Articles:
borneol
Notes:
None found
| Fragrance Demo Formulas | ||
| CAS Number: | 507-70-0 | 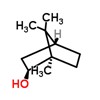 3D/inchi 3D/inchi
|
| Other(deleted CASRN): | 6627-72-1 | |
| ECHA EINECS - REACH Pre-Reg: | 208-080-0 | |
| FDA UNII: | M89NIB437X | |
| Nikkaji Web: | J9.305J | |
| Beilstein Number: | 2038056 | |
| MDL: | MFCD00066426 | |
| CoE Number: | 64 | |
| XlogP3: | 2.70 (est) | |
| Molecular Weight: | 154.25266000 | |
| Formula: | C10 H18 O | |
| BioActivity Summary: | listing | |
| NMR Predictor: | Predict (works with chrome, Edge or firefox) | |
| EFSA/JECFA Comments: | Racemate (±) = DL-Borneol (EFFA, 2010a). CASrn refers to (1R,2S,4R)-rel. Register name to be changed to DL-Borneol (EFFA, 2011m). According to JECFA "Min. Assay value may incl. Isoborneol, other isomers of borneol, trace amounts of fenchyl alcohol & other C10H18O compounds". | |
Category: flavor and fragrance agents
US / EU / FDA / JECFA / FEMA / FLAVIS / Scholar / Patent Information:
| Google Scholar: | Search |
| Google Books: | Search |
| Google Scholar: with word "volatile" | Search |
| Google Scholar: with word "flavor" | Search |
| Google Scholar: with word "odor" | Search |
| Perfumer and Flavorist: | Search |
| Google Patents: | Search |
| US Patents: | Search |
| EU Patents: | Search |
| Pubchem Patents: | Search |
| PubMed: | Search |
| NCBI: | Search |
| JECFA Food Flavoring: | 1385 borneol |
| DG SANTE Food Flavourings: | 02.016 borneol |
| FDA Mainterm (SATF): | 507-70-0 ; BORNEOL |
| FDA Regulation: | |
| FDA PART 172 -- FOOD ADDITIVES PERMITTED FOR DIRECT ADDITION TO FOOD FOR HUMAN CONSUMPTION Subpart F--Flavoring Agents and Related Substances Sec. 172.515 Synthetic flavoring substances and adjuvants. | |
Physical Properties:
| Appearance: | white crystalline powder (est) |
| Assay: | 97.00 to 100.00 % |
| Food Chemicals Codex Listed: | No |
| Melting Point: | 202.00 to 208.00 °C. @ 760.00 mm Hg |
| Boiling Point: | 212.00 to 213.00 °C. @ 760.00 mm Hg |
| Boiling Point: | 135.00 to 136.00 °C. @ 50.00 mm Hg |
| Vapor Pressure: | 0.035000 mmHg @ 25.00 °C. |
| Flash Point: | 150.00 °F. TCC ( 65.56 °C. ) |
| logP (o/w): | 2.690 |
| Soluble in: | |
| alcohol | |
| water, 738 mg/L @ 25 °C (exp) | |
| Insoluble in: | |
| water | |
Organoleptic Properties:
| Odor Type: balsamic | |
| pine woody camphoreous balsamic | |
| Odor Description: at 10.00 % in dipropylene glycol. | pine woody camphor balsamic |
| Odor and/or flavor descriptions from others (if found). | |
Cosmetic Information:
| CosIng: | cosmetic data |
| Cosmetic Uses: |
fragrance perfuming agents |
Suppliers:
Safety Information:
| Preferred SDS: View | |
| European information : | |
| Most important hazard(s): | |
| Xn - Harmful. | |
|
R 22 - Harmful if swallowed. S 02 - Keep out of the reach of children. S 20/21 - When using do not eat, drink or smoke. S 22 - Do not breath dust. S 36/37/39 - Wear suitable clothing, gloves and eye/face protection. | |
| Hazards identification | |
| Classification of the substance or mixture | |
| GHS Classification in accordance with 29 CFR 1910 (OSHA HCS) | |
| None found. | |
| GHS Label elements, including precautionary statements | |
| Pictogram | |
| Hazard statement(s) | |
| None found. | |
| Precautionary statement(s) | |
| None found. | |
| Oral/Parenteral Toxicity: | |
|
oral-rat LD50 500 mg/kg French Demande Patent Document. Vol. #2448856 oral-mouse LD50 1059 mg/kg Shika Gakuho. Journal of Dentistry. Vol. 75, Pg. 934, 1975. oral-rabbit LDLo 2000 mg/kg Reviews of Environmental Contamination and Toxicology. Vol. 113, Pg. 47, 1990. | |
| Dermal Toxicity: | |
| Not determined | |
| Inhalation Toxicity: | |
| Not determined | |
Safety in Use Information:
| Category: | flavor and fragrance agents | ||
| RIFM Fragrance Material Safety Assessment: Search | |||
| IFRA Code of Practice Notification of the 49th Amendment to the IFRA Code of Practice | |||
| Recommendation for dextro,laevo-borneol usage levels up to: | |||
| 3.0000 % in the fragrance concentrate. | |||
| Maximised Survey-derived Daily Intakes (MSDI-EU): | 130.00 (μg/capita/day) | ||
| Maximised Survey-derived Daily Intakes (MSDI-USA): | 23.00 (μg/capita/day) | ||
| Threshold of Concern: | 1800 (μg/person/day) | ||
| Structure Class: | I | ||
| Use levels for FEMA GRAS flavoring substances on which the FEMA Expert Panel based its judgments that the substances are generally recognized as safe (GRAS). | |||
| The Expert Panel also publishes separate extensive reviews of scientific information on all FEMA GRAS flavoring substances and can be found at FEMA Flavor Ingredient Library | |||
| publication number: 3 | |||
| Click here to view publication 3 | |||
| average usual ppm | average maximum ppm | ||
| baked goods: | - | 5.10000 | |
| beverages(nonalcoholic): | 0.25000 | 1.40000 | |
| beverages(alcoholic): | - | - | |
| breakfast cereal: | - | - | |
| cheese: | - | - | |
| chewing gum: | - | 0.30000 | |
| condiments / relishes: | - | - | |
| confectionery froastings: | - | - | |
| egg products: | - | - | |
| fats / oils: | - | - | |
| fish products: | - | - | |
| frozen dairy: | - | 1.40000 | |
| fruit ices: | - | 1.40000 | |
| gelatins / puddings: | - | - | |
| granulated sugar: | - | - | |
| gravies: | - | - | |
| hard candy: | - | 3.70000 | |
| imitation dairy: | - | - | |
| instant coffee / tea: | - | - | |
| jams / jellies: | - | - | |
| meat products: | - | - | |
| milk products: | - | - | |
| nut products: | - | - | |
| other grains: | - | - | |
| poultry: | - | - | |
| processed fruits: | - | - | |
| processed vegetables: | - | - | |
| reconstituted vegetables: | - | - | |
| seasonings / flavors: | - | - | |
| snack foods: | - | - | |
| soft candy: | - | - | |
| soups: | - | - | |
| sugar substitutes: | - | - | |
| sweet sauces: | - | - | |
Safety References:
| Flavor & Extract Manufacturers Association (FEMA) reference(s): | |
| The FEMA GRAS assessment of alicyclic substances used as flavor ingredients. View pdf | |
| European Food Safety Athority(EFSA): | Flavor usage levels; Subacute, Subchronic, Chronic and Carcinogenicity Studies; Developmental / Reproductive Toxicity Studies; Genotoxicity Studies... |
| European Food Safety Authority (EFSA) reference(s): | |
| Flavouring Group Evaluation 47, (FGE.47)[1] - Bicyclic secondary alcohols, ketones and related esters from chemical group 8 - Scientific Opinion of the Panel on Food Additives, Flavourings, Processing Aids and Materials in Contact with Food View page or View pdf | |
| Flavouring Group Evaluation 87, (FGE.87)[1] - Consideration of bicyclic secondary alcohols, ketones and related esters evaluated by JECFA (63rd meeting) structurally related to bicyclic secondary alcohols, ketones and related esters evaluated by EFSA in FGE.47 (2008) - Scientific Opinion of the Panel on Food Additives, Flavourings, Processing Aids and Materials in Contact with Food View page or View pdf | |
| Flavouring Group Evaluation 43: Thujyl alcohol from chemical group 8 View page or View pdf | |
| Scientific Opinion on Flavouring Group Evaluation 87 Revision 1 (FGE.87Rev1): Consideration of bicyclic secondary alcohols, ketones and related esters evaluated by JECFA (63rd meeting) structurally related to bicyclic secondary alcohols, ketones and related esters evaluated by EFSA in FGE.47 (2008) View page or View pdf | |
| Scientific Opinion on Flavouring Group Evaluation 87, Revision 2 (FGE.87Rev2): Consideration of bicyclic secondary alcohols, ketones and related esters evaluated by JECFA (63rd meeting) structurally related to bicyclic secondary alcohols, ketones and related esters evaluated by EFSA in FGE.47Rev1 (2008) View page or View pdf | |
| Safety and efficacy of secondary alicyclic saturated and unsaturated alcohols, ketones, ketals and esters with ketals containing alicyclic alcohols or ketones and esters containing secondary alicyclic alcohols from chemical group 8 when used as flavourings for all animal species View page or View pdf | |
| EPI System: | View |
| Chemical Carcinogenesis Research Information System: | Search |
| AIDS Citations: | Search |
| Cancer Citations: | Search |
| Toxicology Citations: | Search |
| EPA Substance Registry Services (TSCA): | 507-70-0 |
| EPA ACToR: | Toxicology Data |
| EPA Substance Registry Services (SRS): | Registry |
| Laboratory Chemical Safety Summary : | 6552009 |
| National Institute of Allergy and Infectious Diseases: | Data |
| WISER: | UN 1312 |
| WGK Germany: | 3 |
| (1R,2S,4R)-1,7,7-trimethylbicyclo[2.2.1]heptan-2-ol | |
| Chemidplus: | 0000507700 |
| EPA/NOAA CAMEO: | hazardous materials |
| RTECS: | ED7000000 for cas# 507-70-0 |
References:
| Leffingwell: | Chirality or Article |
| (1R,2S,4R)-1,7,7-trimethylbicyclo[2.2.1]heptan-2-ol | |
| NIST Chemistry WebBook: | Search Inchi |
| Canada Domestic Sub. List: | 507-70-0 |
| Pubchem (cid): | 6552009 |
| Pubchem (sid): | 134976836 |
| Flavornet: | 507-70-0 |
| Pherobase: | View |
Other Information:
| (IUPAC): | Atomic Weights of the Elements 2011 (pdf) |
| Videos: | The Periodic Table of Videos |
| tgsc: | Atomic Weights use for this web site |
| (IUPAC): | Periodic Table of the Elements |
| FDA Substances Added to Food (formerly EAFUS): | View |
| CHEBI: | View |
| CHEMBL: | View |
| KEGG (GenomeNet): | C01411 |
| HMDB (The Human Metabolome Database): | HMDB34976 |
| Export Tariff Code: | 2906.19.5000 |
| Typical G.C. | |
| VCF-Online: | VCF Volatile Compounds in Food |
| ChemSpider: | View |
| Wikipedia: | View |
| Formulations/Preparations: grades: technical. | |
Potential Blenders and core components note
Potential Uses:
| agate | FR | |
| balsam | FR | |
| bayberry | FR | |
| bouquet | FR | |
| cardamom oil replacer | FR | |
| carrot | FL | |
| castoreum | FR | |
| cedar | FR | |
| cedar forest | FR | |
| cedarwood | FR | |
| christmas | FR | |
| cinnamon | FR | |
| citronella | FR | |
| coriander | FL/FR | |
| earth | FR | |
| fir | ||
| fir balsam | FR | |
| fir needle oil replacer | FR | |
| frankincense | FR | |
| geranium | FR | |
| ginger | FR | |
| herbal | FR | |
| hollyberry | FR | |
| juniper berry | FR | |
| lavandin | FR | |
| lavender | FR | |
| lavender spike lavender | FL/FR | |
| mace | FR | |
| moss | FR | |
| myrrh | FR | |
| nutmeg | FR | |
| peppermint | FR | |
| pine | FR | |
| sage | FR | |
| spice | FR | |
| spruce | FR | |
| sweet grass | FR | |
| woody | FR |
Occurrence (nature, food, other): note
| achillea tenuifolia lam. flower oil iran @ 0.50% Data GC Search Trop Picture | |
| alpinia galanga oil @ trace% Data GC Search Trop Picture | |
| angelica root oil @ 0.19% Data GC Search Trop Picture | |
| anise seed oil star china @ 0.04% Data GC Search Trop Picture | |
| armoise oil morocco @ 0.7% Data GC Search Trop Picture | |
| artemisia deserti krasch. oil iran @ 0.50% Data GC Search Trop Picture | |
| artemisia diffusa krasch. ex poljak oil iran @ 0.50% Data GC Search Trop Picture | |
| artemisia gypsacea krasch. oil iran @ 2.60% Data GC Search Trop Picture | |
| ayou wood oil @ 2.80% Data GC Search Trop Picture | |
| basil absolute sweet @ 0.20% Data GC Search Trop Picture | |
| calamus leaf oil @ 0.11% Data GC Search Trop Picture | |
| calamus rhizome oil @ 0.11% Data GC Search Trop Picture | |
| calamus root oil @ 0.04% Data GC Search Trop Picture | |
| carrot seed oil spain @ trace-0.35% Data GC Search Trop Picture | |
| cascarilla bark oil @ 0.94% Data GC Search Trop Picture | |
| castoreum Search PMC Picture | |
| chamomile oil @ 0.20% Data GC Search Trop Picture | |
| chamomile oil morocco @ 1.00% Data GC Search Trop Picture | |
| champaca concrete @ 0.10% Data GC Search Trop Picture | |
| cinnamon fruit oil india @ 0.3-0.4% Data GC Search Trop Picture | |
| cistus oil @ 2.0% Data GC Search Trop Picture | |
| citronella oil ceylon @ 5.23% Data GC Search Trop Picture | |
| citronella oil china @ 0.06% Data GC Search Trop Picture | |
| citronella oil java @ 0.05% Data GC Search Trop Picture | |
| citronella oil zimbabwe @ 0.90% Data GC Search Trop Picture | |
| clary sage oil spain @ 0.12% Data GC Search Trop Picture | |
| coriander seed oil @ 0.30% Data GC Search Trop Picture | |
| coriander seed oil CO2 extract @ 0.07% Data GC Search Trop Picture | |
| coriander seed oil cuba @ 1.55% Data GC Search Trop Picture | |
| croton cajucara benth. leaf oil brazil @ trace% Data GC Search Trop Picture | |
| croton flavens l. (welensali) leaf oil curacao @ 1.20% Data GC Search Trop Picture | |
| curcuma amada roxb. rhizome oil india @ 1.30% Data GC Search Trop Picture | |
| cypress cone oil egypt @ 0.3% Data GC Search Trop Picture | |
| cypress oil @ trace% Data GC Search Trop Picture | |
| davana oil @ 0.5% Data GC Search Trop Picture | |
| erigeron graveolens l. oil iran @ 60.70% Data GC Search Trop Picture | |
| eucalyptus camaldulensis dehn. leaf oil jerusalem @ 3.80% Data GC Search Trop Picture | |
| eucalyptus globulus bicostata oil @ 0.09% Data GC Search Trop Picture | |
| eucalyptus globulus oil @ 0.06% Data GC Search Trop Picture | |
| eucalyptus globulus oil burundia @ 0.20% Data GC Search Trop Picture | |
| eucalyptus globulus oil pakistan @ 0.20% Data GC Search Trop Picture | |
| eucalyptus globulus pseudoglobulus oil @ 0.07% Data GC Search Trop Picture | |
| featherfew leaf oil @ 0.60% Data GC Search Trop Picture | |
| feverfew leaf oil belgium @ 1.0% Data GC Search Trop Picture | |
| fir needle oil canada @ 0.31-2.07% Data GC Search Trop Picture | |
| fir needle oil siberia @ 1.66% Data GC Search Trop Picture | |
| geranium rose-scented oil (pelargonium spp.) cuba @ 0.10% Data GC Search Trop Picture | |
| ginger root oil brazil @ 0.70% Data GC Search Trop Picture | |
| ginger root oil china @ 0.48% Data GC Search Trop Picture | |
| ginger root oil CO2 extract @ 0.60% Data GC Search Trop Picture | |
| ginger root oil CO2 extract australia @ 0.60% Data GC Search Trop Picture | |
| glycosmis pentaphylla (cor.) seed oil india @ 0.40% Data GC Search Trop Picture | |
| guava fruit headspace reunion @ 0.50% Data GC Search Trop Picture | |
| hinoki leaf oil @ 1.23% Data GC Search Trop Picture | |
| hinoki root oil @ 0.54% Data GC Search Trop Picture | |
| hinoki wood oil @ 0.33% Data GC Search Trop Picture | |
| ho leaf oil @ 0.16% Data GC Search Trop Picture | |
| hypericum dogonbadanicum assadi oil iran @ 0.70% Data GC Search Trop Picture | |
| kachur oil @ 4.90% Data GC Search Trop Picture | |
| kewda oil @ 0.17-0.20% Data GC Search Trop Picture | |
| labdanum leaf oil @ 0.80% Data GC Search Trop Picture | |
| labdanum oil @ 1.45% Data GC Search Trop Picture | |
| laurel leaf oil turkey @ 0.30% Data GC Search Trop Picture | |
| lavandin absolute grosso @ 1.50% Data GC Search Trop Picture | |
| lavandin oil abrialis @ 3.65% Data GC Search Trop Picture | |
| lavandin oil china @ 0.89% Data GC Search Trop Picture | |
| lavandin oil grosso @ 2.89% Data GC Search Trop Picture | |
| lavandin water (lavandula hydrida) @ 1.35% Data GC Search Trop Picture | |
| lavandula officinalis flower oil @ 1.60% Data GC Search Trop Picture | |
| lavender oil @ 1.85% Data GC Search Trop Picture | |
| lavender oil CO2 extract france @ 2.30% Data GC Search Trop Picture | |
| lavender oil spike @ 2.50% Data GC Search Trop Picture | |
| lavender oil spike france @ 1.69% Data GC Search Trop Picture | |
| lavender oil spike spain @ 1.13% Data GC Search Trop Picture | |
| lavender spike water @ 2.26% Data GC Search Trop Picture | |
| lavender water bulgaria @ 2.20% Data GC Search Trop Picture | |
| layana oil kenya @ 5.09% Data GC Search Trop Picture | |
| layana oil wild zimbabwe @ 0.60-3.40% Data GC Search Trop Picture | |
| layana oil zimbabwe @ 2.8% Data GC Search Trop Picture | |
| lemon oil california @ 0.01% Data GC Search Trop Picture | |
| lemongrass oil @ 0.05% Data GC Search Trop Picture | |
| lime oil CO2 extract mexico @ 3.25% Data GC Search Trop Picture | |
| lime oil distilled peru @ 0.82% Data GC Search Trop Picture | |
| lime oil expressed florida @ trace% Data GC Search Trop Picture | |
| lippia aff. gracillis h.b.k. leaf oil brazil @ 0.50% Data GC Search Trop Picture | |
| mace oil east india @ 0.16% Data GC Search Trop Picture | |
| mangrove bark red oil cuba @ trace% Data GC Search Trop Picture | |
| marjoram oil @ 0.52% Data GC Search Picture | |
| marjoram oil spain @ trace% Data GC Search Trop Picture | |
| marjoram oil sweet turkey @ 0.00-0.64% Data GC Search Trop Picture | |
| mastic fruit oil @ 1.44% Data GC Search Trop Picture | |
| mastic leaf oil @ 0.10% Data GC Search Trop Picture | |
| mastic oil @ 0.12% Data GC Search Trop Picture | |
| myrtle lemon scented leaf oil australia @ 0.10% Data GC Search Trop Picture | |
| nutmeg extract CO2 west indian @ trace% Data GC Search Trop Picture | |
| nutmeg flower oil @ 0.14% Data GC Search Trop Picture | |
| nutmeg leaf oil @ 0.00-0.09% Data GC Search Trop Picture | |
| nutmeg oil india @ 0.25% Data GC Search Trop Picture | |
| oregano oil mexico @ 0.09% Data GC Search Trop Picture | |
| origanum oil greece @ trace% Data GC Search Trop Picture | |
| peppermint oil mongolia @ 0.05% Data GC Search Trop Picture | |
| petitgrain bergamot oil @ 0.02% Data GC Search Trop Picture | |
| phoebe oil brazil @ 0.01% Data GC Search Trop Picture | |
| pine needle oil scotch siberia @ 0.4-0.7% Data GC Search Trop Picture | |
| pinus sylvestris l. needle oil estonia @ 0.30% Data GC Search Trop Picture | |
| pinus sylvestris l. needle oil yugoslavia @ 0.10% Data GC Search Trop Picture | |
| ravintsara leaf oil @ 0.21%% Data GC Search Picture | |
| rosemary oil china @ 1.71% Data GC Search Trop Picture | |
| rosemary oil CO2 extract @ 15.56% Data GC Search Trop Picture | |
| rosemary oil corsica @ 3.20% Data GC Search Trop Picture | |
| rosemary oil egypt @ 1.69% Data GC Search Trop Picture | |
| rosemary oil france @ 3.24-6.53% Data GC Search Trop Picture | |
| rosemary oil morocco @ 3.00-4.51% Data GC Search Trop Picture | |
| rosemary oil spain @ 9.0% Data GC Search Trop Picture | |
| rosemary oil turkey @ 17.50% Data GC Search Trop Picture | |
| sage oil albania @ 8.70% Data GC Search Trop Picture | |
| sage oil cuba @ 3.81% Data GC Search Trop Picture | |
| sage oil dalmatian @ 1.20% Data GC Search Trop Picture | |
| sage oil dalmatian @ 2.95% Data GC Search Trop Picture | |
| sage oil dalmatian @ 8.40% Data GC Search Trop Picture | |
| sage oil england @ 4.10% Data GC Search Trop Picture | |
| sage oil germany @ 3.12% Data GC Search Trop Picture | |
| sage oil hydrodistillation france @ 1.90% Data GC Search Trop Picture | |
| sage oil lithuania @ 2.04% Data GC Search Trop Picture | |
| sage oil sardinia @ 3.00% Data GC Search Trop Picture | |
| sage oil spain @ 0.8% Data GC Search Trop Picture | |
| sage oil spain @ 1.5-6.4% Data GC Search Trop Picture | |
| sage oil spain @ 2.10% Data GC Search Trop Picture | |
| salvia lavandulifolia vahl. leaf oil @ 1.50% Data GC Search Trop Picture | |
| salvia lavandulifolia vahl. leaf oil spain @ 2.20% Data GC Search Trop Picture | |
| salvia officinalis oil cuba @ 3.81% Data GC Search Trop Picture | |
| salvia officinalis oil reunion @ 1.87% Data GC Search Trop Picture | |
| salvia officinalis seed oil tunisia @ 3.54% Data GC Search Trop Picture | |
| salvia plebeia oil @ 0.55% Data GC Search Trop Picture | |
| salvia sclarea oil @ 0.64% Data GC Search Trop Picture | |
| santolina oil @ 4.78% Data GC Search Trop Picture | |
| savory oil winter @ 1.60% Data GC Search Trop Picture | |
| savory oleoresin winter @ 1.60% Data GC Search Trop Picture | |
| snake root oil canada @ 0.10% Data GC Search Trop Picture | |
| tanacetum annum l. oil marocco @ 2.70% Data GC Search Trop Picture | |
| tansy flower oil canada @ 12.49% Data GC Search Trop Picture | |
| tansy oil marocco @ 2.10% Data GC Search Trop Picture | |
| thyme oil portuguese @ 1.0% Data GC Search Trop Picture | |
| thyme oil red CO2 extract @ 0.02% Data GC Search Trop Picture | |
| thyme oil red spain @ 0.44-0.45% Data GC Search Trop Picture | |
| thyme oil spain @ 1.63% Data GC Search Trop Picture | |
| thyme oil spain @ 4.06% Data GC Search Trop Picture | |
| thyme oil wild or creeping finland @ 0.20% Data GC Search Trop Picture | |
| thyme oil wild or creeping pakistan @ 3.13% Data GC Search Trop Picture | |
| thymus richardii pers. subsp. nitidus (guss.) jalas oil italy @ trace% Data GC Search Trop Picture | |
| turmeric oil china @ 0.02% Data GC Search Trop Picture | |
| turmeric root oil CO2 extract @ 1.08% Data GC Search Trop Picture | |
| valerian root oil @ 0.1-0.6% Data GC Search Trop Picture | |
| valerian root oil CO2 extract china @ 0.47% Data GC Search Trop Picture | |
| wormwood oil annus france @ 0.00-0.16% Data GC Search Trop Picture | |
| wormwood oil italy @ trace% Data GC Search Trop Picture | |
| yarrow oil @ 2.50% Data GC Search Trop Picture | |
| yarrow oil hungary @ 0.30% Data GC Search Trop Picture | |
| zingiber officinale root oil china @ 0.20% Data GC Search Trop Picture | |
| zingiber zerumbet (l.) sm. root oil india @ 4.90% Data GC Search Trop Picture |
Synonyms:
| bicyclo(2.2.1)heptan-2-ol, 1,7,7-trimethyl-, (1R,2S,4R)-rel- | |
| bicyclo[2.2.1]heptan-2-ol, 1,7,7-trimethyl-, (1R,2S,4R)- | |
| borneol | |
| (1R,2S,4R)- | borneol |
| dextro,laevo- | borneol |
| DL- | borneol |
| N- | borneol |
| borneol 60/40 | |
| borneol crystals | |
| borneol flakes | |
| borneol natural | |
| bhimsaim | camphor |
| borneo | camphor |
| dextro,laevo-1,7,7- | trimethyl bicyclo(2.2.1)heptan-2-ol |
| DL-1,7,7- | trimethyl bicyclo(2.2.1)heptan-2-ol |
| (1R-endo)-1,7,7- | trimethylbicyclo(2.2.1)heptan-2-ol |
| (1R,2S,4R)-rel-1,7,7- | trimethylbicyclo(2.2.1)heptan-2-ol |
| (1R-endo)-1,7,7- | trimethylbicyclo[2.2.1]heptan-2-ol |
| (1R,2S,4R)-1,7,7- | trimethylbicyclo[2.2.1]heptan-2-ol |



















