|
Category: flavor and fragrance agents
US / EU / FDA / JECFA / FEMA / FLAVIS / Scholar / Patent Information:
Physical Properties:
| Appearance: | colorless to pale yellow clear liquid (est) |
| Assay: | 85.00 to 100.00 % sum of isomers
|
| Food Chemicals Codex Listed: | Yes |
| Specific Gravity: | 0.85000 to 0.85800 @ 25.00 °C.
|
| Pounds per Gallon - (est).: | 7.073 to 7.139
|
| Specific Gravity: | 0.85100 to 0.85900 @ 20.00 °C.
|
| Pounds per Gallon - est.: | 7.089 to 7.156
|
| Refractive Index: | 1.44600 to 1.45300 @ 20.00 °C.
|
| Optical Rotation: | -1.00 to -11.00
|
| Boiling Point: | 206.00 to 207.00 °C. @ 760.00 mm Hg
|
| Boiling Point: | 106.00 to 108.00 °C. @ 15.00 mm Hg
|
| Acid Value: | 3.00 max. KOH/g
|
| Vapor Pressure: | 0.280000 mmHg @ 25.00 °C. |
| Vapor Density: | 5.3 ( Air = 1 ) |
| Flash Point: | 169.00 °F. TCC ( 76.11 °C. )
|
| logP (o/w): | 3.297 (est) |
| Shelf Life: | 12.00 month(s) or longer if stored properly. |
| Storage: | store in cool, dry place in tightly sealed containers, protected from heat and light. |
| Soluble in: |
| | alcohol | | | fixed oils | | | water, 38.94 mg/L @ 25 °C (est) |
| Insoluble in: |
| | water | | | glycerin |
| Stability: |
| | non-discoloring |
Organoleptic Properties:
| |
| Odor Type: floral |
| |
| Odor Strength: | high ,
recommend smelling in a 10.00 % solution or less |
| |
| Substantivity: | 16 hour(s) at 100.00 % |
| |
| | sweet dry floral herbal waxy aldehydic citrus |
Odor Description:
at 10.00 % in dipropylene glycol. | sweet dry floral herbal waxy aldehydic citrus
Luebke, William tgsc, (1984) |
| |
| | sweet floral rose waxy citrus green |
Odor Description:
| Sweet, floral rosy waxy and citrus green
Mosciano, Gerard P&F 14, No. 6, 47, (1989) |
| |
| |
| Flavor Type: floral |
| |
| | floral green rose citrus lemon |
Taste Description:
at 10.00 ppm. | Floral, green, rosy and citrus lemon
Mosciano, Gerard P&F 14, No. 6, 47, (1989) |
| |
| Odor and/or flavor descriptions from others (if found). |
| |
| Takasago |
| Citronellal Natural ≥85% as Citronellal |
| Odor Description: | Powerful, fresh, green-citrusy, slightly woody
Mainly used in citrus-lemongrass type fragrances. |
| |
| Moellhausen |
| CITRONELLAL |
| Odor Description: | fresh, citrus, floreal |
| Taste Description: | green, aldehyde, fruity |
| |
| PerfumersWorld |
| Citronellal |
| Odor Description: | fresh citrusy-green fruity-floral-herb
BLENDS WITH - Citrus-notes |
| |
| Prodasynth |
| CITRONELLAL (SUM OF ISOMERS > 96%) |
| Odor Description: | CITRUS, CITRONELLA, HERBAL, ROSE |
| |
| |
Cosmetic Information:
Suppliers:
| Advanced Biotech |
| CITRONELLAL NATURAL
85% min. |
| Ambles Nature et Chimie |
| CITRONELLAL NAT
|
| Augustus Oils |
| Citronellal
|
| Services |
| Axxence Aromatic |
| CITRONELLAL Natural
Kosher |
| Sustainability |
| BASF |
| Citronellal
Odor: Citrus, green, fruity, rose Use: It shows an intensive citrus-like odor but seems to be less sweet and fruity than Citral. The quality of this Aroma Chemical is very good and comparable to Citronella oil. Flavor: Pungent, fresh, green, citrus, slightly woody Due to the rather unstable character of this aldehyde and intensity it is generally only used in traces for citrus flavors. |
| Beijing Lys Chemicals |
| Citronellal
|
| Berjé |
| Citronellal 85/90% ex Eucalyptus Citriodora
|
| Media |
| Berjé |
| Citronellal Synthetic
|
| BOC Sciences |
| For experimental / research use only. |
| Citronellal
|
| Charkit Chemical |
| CITRONELLAL FEMA 2307
|
| Citrus and Allied Essences |
| Citronellal 85/90% FCC (natural)
|
| Market Report |
| Diffusions Aromatiques |
| CITRONELLAL NATUREL
|
| ECSA Chemicals |
| CITRONELLALE NATURAL
|
| ECSA TRADE THE MOST UPDATED FINANCIAL PUBLICATION ON THE WORLD OF CHEMISTRY |
| ECSA Chemicals |
| CITRONELLALE
|
| EMD Millipore |
| For experimental / research use only. |
| Citronellal
|
| Ernesto Ventós |
| CITRONELLAL, NATURAL EX-CITRIODORA
|
| Ernesto Ventós |
| CITRONELLAL, NATURAL EX-CITRONELLA
|
| Ernesto Ventós |
| CITRONELLAL
|
| Excellentia International |
| Citronellal Natural
|
| Fleurchem |
| citronellal natural
|
| Fleurchem |
| citronellal
|
| Foreverest Resources |
| Citronellal
|
| Fuzhou Farwell |
| Citronellal
|
| George Uhe Company |
| Citronellal 85%
Available in FCC |
| Global Essence |
| Citronellal
|
| HDDES Group |
| Citronellal
Natural |
| Indukern F&F |
| CITRONELLAL NATURAL
Odor: CITRUS, LEMON, GREEN, ROSE |
| Indukern F&F |
| CITRONELLAL
Odor: CITRUS, LEMON, GREEN, WOODY |
| K.L. Koh Enterprise |
| CITRONELLAL
|
| Kanta Enterprises |
| Citronellal
|
| Lluch Essence |
| CITRONELLAL NAT. EX-EUCAL. CITRIODORA
|
| Lluch Essence |
| CITRONELLAL NATURAL EX-CITRONELLA 85%
|
| Lluch Essence |
| CITRONELLAL SYNTH. 95%
|
| Lluch Essence |
| CITRONELLAL SYNTH. 96%
|
| M&U International |
| CITRONELLAL
|
| Moellhausen |
| CITRONELLAL NAT.
|
| Moellhausen |
| CITRONELLAL
Odor: fresh, citrus, floreal Flavor: green, aldehyde, fruity |
| Nagar Haveli Perfumes & Aromatics |
| Citronellal
Natural Odor: Sweet, dry, floral, herbal, waxy, aldehydic citrus, green, rosy and citrus lemon |
| Nippon Terpene Chemicals |
| Citronellal 95%
|
| Payand Betrand |
| Citronellal China, Natural Isolated Constituent
|
| Penta International |
| CITRONELLAL FCC NATURAL
|
| Penta International |
| CITRONELLAL FCC SYNTHETIC
|
| PerfumersWorld |
| Citronellal
Odor: fresh citrusy-green fruity-floral-herb Use: BLENDS WITH - Citrus-notes |
| Perfumery Laboratory |
| Citronellal
Odor: Powerful, fresh, citrus with green notes |
| Phoenix Aromas & Essential Oils |
| Citronellal
|
| Privi Organics |
| Citronellal
|
| Prodasynth |
| CITRONELLAL
(SUM OF ISOMERS > 96%) Odor: CITRUS, CITRONELLA, HERBAL, ROSE |
| R C Treatt & Co Ltd |
| Citronellal Natural
|
| Reincke & Fichtner |
| Citronellal natural
|
| Reincke & Fichtner |
| Citronellal
|
| Robertet |
| Citronellal naturel
|
| Seasons and Harvest / Crop calendar |
| Sigma-Aldrich |
| (±)-citronellal, ≥85%, FG
Odor: cherry; lemon; green; rose; sweet |
| Certified Food Grade Products |
| Sigma-Aldrich |
| (±)-Citronellal, natural, ≥85%, FCC, FG
Odor: cherry; lemon; green; rose; sweet |
| Som Extracts |
| CITRONELLAL
|
| SRS Aromatics |
| CITRONELLAL EXTRA (FG)
Odor: Sweet, Floral, Rosy, Waxy, Citrus Green |
| SRS Aromatics |
| CITRONELLAL NATURAL
|
| SRS Aromatics |
| CITRONELLAL
|
| The Lermond Company |
| CITRONELLAL
|
| The Perfumers Apprentice |
| Citronellal (Natural)
Odor: Powerful fresh green citrusy, slight woody odor/musky rose |
| The Perfumers Apprentice |
| Citronellal
Odor: Powerful fresh green citrusy, slight woody odor/musky rose |
| Van Aroma |
| CITRONELLAL NATURAL MIN 85%
Odor: Fresh and sweet |
| maps.vanaroma.com |
| Van Aroma |
| CITRONELLAL NATURAL MIN 90%
|
| Van Aroma |
| CITRONELLAL NATURAL MIN 95%
|
| Vigon International |
| Citronellal FCC Natural
Odor: INTENSE, LEMON-CITRONELLA-ROSE |
| Vigon International |
| Citronellal Synthetic
Odor: Citrus, green, fruity, rose |
| WholeChem |
| Citronellal
|
| Xiamen Doingcom Chemical |
| For experimental / research use only. |
| Citronellal Ex-Citronella Oil
|
| Xiamen Doingcom Chemical |
| For experimental / research use only. |
| Citronellal Ex-Eucalyptus Citriodara Oil
|
| Zanos |
| CITRONELLAL (natural isolated constituent)
|
| Zanos |
| Citronellal
|
Safety Information:
| Preferred SDS: View |
| European information : |
| Most important hazard(s): | | Xn N - Harmful, Dangerous for the environment. |
R 43 - May cause sensitisation by skin contact.
R 63 - Possible risk of harm to the unborn child.
S 02 - Keep out of the reach of children.
S 23 - Do not breath vapour.
S 26 - In case of contact with eyes, rinse immediately with plenty of water and seek medical advice.
S 36/37/39 - Wear suitable clothing, gloves and eye/face protection.
S 45 - In case of accident or if you feel unwell seek medical advice immediately.
|
| |
| Hazards identification |
| |
| Classification of the substance or mixture |
| GHS Classification in accordance with 29 CFR 1910 (OSHA HCS) |
| None found. |
| GHS Label elements, including precautionary statements |
| |
| Pictogram | |
| |
| Hazard statement(s) |
| None found. |
| Precautionary statement(s) |
| None found. |
| Human Experience: |
| 4 % solution: no irritation or sensitization. |
| Oral/Parenteral Toxicity: |
oral-rat LD50 2420 mg/kg
National Technical Information Service. Vol. OTS0557726
intraperitoneal-mouse LD50 > 200 mg/kg
BEHAVIORAL: SOMNOLENCE (GENERAL DEPRESSED ACTIVITY)
LUNGS, THORAX, OR RESPIRATION: DYSPNEA
National Technical Information Service. Vol. OTS0535480
|
| Dermal Toxicity: |
skin-rabbit LD50 > 2500 mg/kg
Food and Cosmetics Toxicology. Vol. 13, Pg. 755, 1975.
|
| Inhalation Toxicity: |
|
Not determined
|
Safety in Use Information:
| Category: | flavor and fragrance agents |
| RIFM Fragrance Material Safety Assessment: Search |
| IFRA Code of Practice Notification of the 49th Amendment to the IFRA Code of Practice |
| IFRA Critical Effect: | Dermal sensitization and systemic toxicity |
| IFRA: | View Standard |
| View IFRA Standards Library for complete information. |
| Please review Amendment 49 IFRA documentation for complete information. |
| IFRA RESTRICTION LIMITS IN THE FINISHED PRODUCT (%): |
| Category 1: Products applied to the lips |
| 0.41 % |
| Category 2: Products applied to the axillae |
| 0.16 % |
| Category 3: Products applied to the face/body using fingertips |
| 0.026 % |
| Category 4: Products related to fine fragrance |
| 0.49 % |
| | Category 5: Products applied to the face and body using the hands (palms), primarily leave-on |
| Category 5A: Body lotion products applied to the body using the hands (palms), primarily leave-on |
| 0.33 % |
| Category 5B: Face moisturizer products applied to the face using the hands (palms), primarily leave-on |
| 0.051 % |
| Category 5C: Hand cream products applied to the hands using the hands (palms), primarily leave-on |
| 0.10 % |
| Category 5D: Baby Creams, baby Oils and baby talc |
| 0.017 % |
| Category 6: Products with oral and lip exposure |
| 0.82 % |
| | Category 7: Products applied to the hair with some hand contact |
| Category 7A: Rinse-off products applied to the hair with some hand contact |
| 0.077 % |
| Category 7B: Leave-on products applied to the hair with some hand contact |
| 0.077 % |
| Category 8: Products with significant anogenital exposure |
| 0.017 % |
| Category 9: Products with body and hand exposure, primarily rinse off |
| 1.40 % |
| | Category 10: Household care products with mostly hand contact |
| Category 10A: Household care excluding aerosol products (excluding aerosol/spray products) |
| 1.40 % |
| Category 10B: Household aerosol/spray products |
| 2.30 % |
| | Category 11: Products with intended skin contact but minimal transfer of fragrance to skin from inert substrate |
| Category 11A: Products with intended skin contact but minimal transfer of fragrance to skin from inert substrate without UV exposure |
| 0.017 % |
| Category 11B: Products with intended skin contact but minimal transfer of fragrance to skin from inert substrate with potential UV exposure |
| 0.017 % |
| Category 12: Products not intended for direct skin contact, minimal or insignificant transfer to skin |
| No Restriction |
| | Notes: |
| IFRA FLAVOR REQUIREMENTS: |
Due to the possible ingestion of small amounts of fragrance ingredients from their use in products in Categories 1 and 6, materials must not only comply with IFRA Standards but must also be recognized as safe as a flavoring ingredient as defined by the IOFI Code of Practice (www.iofi.org). For more details see chapter 1 of the Guidance for the use of IFRA Standards. |
| |
| Maximised Survey-derived Daily Intakes (MSDI-EU): | 810.00 (μg/capita/day) |
| Maximised Survey-derived Daily Intakes (MSDI-USA): | 324.00 (μg/capita/day) |
| Threshold of Concern: | 1800 (μg/person/day) |
| Structure Class: | I |
| Use levels for FEMA GRAS flavoring substances on which the FEMA Expert Panel based its judgments that the substances are generally recognized as safe (GRAS). |
| The Expert Panel also publishes separate extensive reviews of scientific information on all FEMA GRAS flavoring substances and can be found at FEMA Flavor Ingredient Library |
| publication number: 3 |
| Click here to view publication 3 |
| | average usual ppm | average maximum ppm |
| baked goods: | - | 4.70000 |
| beverages(nonalcoholic): | - | 4.00000 |
| beverages(alcoholic): | - | - |
| breakfast cereal: | - | - |
| cheese: | - | - |
| chewing gum: | - | 0.30000 |
| condiments / relishes: | - | - |
| confectionery froastings: | - | - |
| egg products: | - | - |
| fats / oils: | - | - |
| fish products: | - | - |
| frozen dairy: | - | 1.30000 |
| fruit ices: | - | 1.30000 |
| gelatins / puddings: | - | 0.60000 |
| granulated sugar: | - | - |
| gravies: | - | - |
| hard candy: | - | 4.50000 |
| imitation dairy: | - | - |
| instant coffee / tea: | - | - |
| jams / jellies: | - | - |
| meat products: | - | - |
| milk products: | - | - |
| nut products: | - | - |
| other grains: | - | - |
| poultry: | - | - |
| processed fruits: | - | - |
| processed vegetables: | - | - |
| reconstituted vegetables: | - | - |
| seasonings / flavors: | - | - |
| snack foods: | - | - |
| soft candy: | - | - |
| soups: | - | - |
| sugar substitutes: | - | - |
| sweet sauces: | - | - |
Safety References:
| European Food Safety Athority(EFSA): | Flavor usage levels; Subacute, Subchronic, Chronic and Carcinogenicity Studies; Developmental / Reproductive Toxicity Studies; Genotoxicity Studies... |
| European Food Safety Authority (EFSA) reference(s): |
Flavouring Group Evaluation 3, Revision 1 (FGE.03Rev1): Acetals of branched- and straight-chain aliphatic saturated primary alcohols and branched- and straight-chain saturated or unsaturated aldehydes, an ester of a hemiacetal and an orthoester of formic acid, from chemical groups 1, 2 & 4 Commission Regulation (EC) No 1565/2000 of 18 July 2000) [1] - Scientific Opinion of the Scientific Panel on Food Additives, Flavourings, Processing Aids and Materials in contact with Food (AFC)on a request from the Commission
View page or View pdf |
Flavouring Group Evaluation 72 (FGE.72): Consideration of aliphatic, branched-chain saturated and unsaturated alcohols, aldehydes, acids, and related esters evaluated by the JECFA (61st meeting) structurally related to branched- and straight-chain unsaturated carboxylic acids. Esters of these and straight-chain aliphatic saturated alcohols evaluated by EFSA in FGE.05Rev2 (2010)
View page or View pdf |
Scientific Opinion on Flavouring Group Evaluation 95 (FGE.95): Consideration of aliphatic, linear or branched-chain saturated and unsaturated alcohols, aldehydes, acids and related esters evaluated by JECFA (69th meeting) structurally related to esters of branched- and straight-chain aliphatic saturated primary alcohols and of one secondary alcohol, and branched- and straight-chain unsaturated carboxylic acids evaluated by EFSA in FGE.05Rev1 (2008)
View page or View pdf |
Scientific Opinion on Flavouring Group Evaluation 72, Revision 1 (FGE.72Rev1): Consideration of aliphatic, branched-chain saturated and unsaturated alcohols, aldehydes, acids, and related esters evaluated by the JECFA (61st meeting) structurally related to branched- and straight-chain unsaturated carboxylic acids, esters of these and straight-chain aliphatic saturated alcohols evaluated by EFSA in FGE.05Rev2
View page or View pdf |
Safety and efficacy of non-conjugated and accumulated unsaturated straight-chain and branched-chain, aliphatic primary alcohols, aldehydes, acids, acetals and esters belonging to chemical group 4 when used as flavourings for all animal species
View page or View pdf |
Scientific Opinion on Flavouring Group Evaluation 72, Revision 2 (FGE.72Rev2): consideration of aliphatic, branched-chain saturated and unsaturated alcohols, aldehydes, acids and related esters evaluated by JECFA (61st, 68th and 69th meetings) and structurally related to flavouring substances in FGE.05Rev3
View page or View pdf |
| EPI System: | View |
| Chemical Carcinogenesis Research Information System: | Search |
| AIDS Citations: | Search |
| Cancer Citations: | Search |
| Toxicology Citations: | Search |
| EPA Substance Registry Services (TSCA): | 106-23-0 |
| EPA ACToR: | Toxicology Data |
| EPA Substance Registry Services (SRS): | Registry |
| Laboratory Chemical Safety Summary : | 7794 |
| National Institute of Allergy and Infectious Diseases: | Data |
| WISER: | UN 3082 |
| WGK Germany: | 1 |
| | 3,7-dimethyloct-6-enal |
| Chemidplus: | 0000106230 |
| RTECS: | RH2140000 for cas# 106-23-0 |
References:
Other Information:
Potential Blenders and core components note
| |
| For Odor |
| aldehydic |
| aldehydic |
| | acetyl nonyryl | FL/FR |
| | aldehydic fragrance | FR |
| | citronellyl oxyacetaldehyde | FL/FR |
| | decanal (aldehyde C-10) | FL/FR |
| 9- | decenal | FL/FR |
| | dodecanal (aldehyde C-12 lauric) | FL/FR |
| (Z)-4- | dodecenal | FL/FR |
| | geranyl oxyacetaldehyde | FR |
| 3- | methyl-4-(1-methyl hexyl oxy) butyraldehyde | FR |
| 3- | methyl-4-heptyl oxybutyraldehyde | FR |
| | muguet undecadienal | FR |
| | nonanal (aldehyde C-9) | FL/FR |
| | nonanal diethyl acetal | FL/FR |
| | octanal (aldehyde C-8) | FL/FR |
| | TMH aldehyde | FR |
| N/i- | tridecanal | FR |
| 2- | tridecenal | FL/FR |
| | undecanal | FL/FR |
| N-/i- | undecanal | FR |
| (Z)-8- | undecenal | FR |
| 10- | undecenal (aldehyde C-11 undecylenic) | FL/FR |
| balsamic |
| | benzyl benzoate | FL/FR |
| | benzyl salicylate | FL/FR |
| | cinnamyl alcohol | FL/FR |
| | cyclohexyl benzoate | FL/FR |
| burnt |
| | lepidine | FL/FR |
| citrus |
| | acetaldehyde citronellyl methyl acetal | FR |
| | aldehydic nitrile | FR |
| | aldenal C-10 | FR |
| | bergamot oil bergaptene reduced italy | FL/FR |
| | bergamot oil turkey | FL/FR |
| beta- | bisabolol | FL/FR |
| | citral diethyl acetal | FL/FR |
| | citral dimethyl acetal | FL/FR |
| | citronellyl nitrile | FR |
| | citrus limon peel oil expressed | FL/FR |
| | citrus paradisi peel extract | FL/FR |
| | curacao specialty | FR |
| (Z)-4- | decenal | FL/FR |
| (E)-4- | decenal | FL/FR |
| (Z)-7- | decenal | FR |
| | jambu flower oil brazil | |
| | lemon oil c.p. california | FL/FR |
| | lemon oil c.p. furocoumarin reduced | FL/FR |
| | lemongrass oil | FL/FR |
| | lime octadienal | FR |
| (Z)- | linalool oxide (pyranoid) | FL/FR |
| | mandarin specialty | FR |
| | marine decadienal | FR |
| | methyl heptenone | FL/FR |
| alpha- | methylene citronellal | FR |
| | nonanal dimethyl acetal | FL/FR |
| | orange fruit oil | FL/FR |
| blood | orange oil italy | FL/FR |
| | tetrahydrocitral | FL/FR |
| | verbena absolute france | FL/FR |
| | verbena oil terpeneless | FL/FR |
| | waxy nitrile | FR |
| earthy |
| | octyl phenyl acetate | FL/FR |
| fatty |
| 2,4- | decadien-1-ol | FL/FR |
| 2- | decen-1-ol | FL/FR |
| | methyl 10-undecenoate | FL/FR |
| (E)-2- | nonenal | FL/FR |
| floral |
| ortho- | acetyl-para-cresol | FL/FR |
| iso | amyl salicylate | FL/FR |
| | amyl salicylate | FL/FR |
| iso | amyl undecylenate | FL/FR |
| | benzyl alcohol | FL/FR |
| | benzyl pivalate | FL/FR |
| | bois de rose oil brazil | FL/FR |
| | butyl benzyl ether | FL/FR |
| | butyl tiglate | FR |
| | cassie absolute replacer | FL/FR |
| | cassis cyclohexene | FR |
| | citronellol | FL/FR |
| (S)- | citronellyl acetate | FL/FR |
| | citronellyl butyrate | FL/FR |
| | citronellyl ethyl ether | FR |
| | citronellyl phenyl acetate | FL/FR |
| | citronellyl propionate | FL/FR |
| | citrus propanol | FR |
| | coranol (Firmenich) | FR |
| | cymbopogon validus leaf oil | FR |
| (Z)-alpha- | damascone | FL/FR |
| gamma- | damascone | FR |
| 9- | decen-1-ol | FL/FR |
| (Z)-4- | decen-1-yl acetate | FL/FR |
| | dihydrocarvyl acetate | FL/FR |
| | dihydrocitronellyl ethyl ether | FR |
| | dihydrojasmone | FL/FR |
| | dihydrolinalool | FL/FR |
| | dihydromyrcene | FR |
| | dimethyl benzyl carbinyl butyrate | FL/FR |
| | dimethyl octanol | FL/FR |
| 2,4- | dimethyl-3-cyclohexene-1-methanol | FR |
| 3,6- | dimethyl-3-octanol | FL/FR |
| | eau de brouts absolute | FR |
| | ethyl linalool | FR |
| | ethyl linalyl acetal | FR |
| | ethyl linalyl acetate | FR |
| | ethyl linalyl ether | FL/FR |
| | farnesyl acetate | FL/FR |
| | floral pyran | FR |
| | gelsone (IFF) | FL/FR |
| | geraniol | FL/FR |
| | geranium rose-scented oil cuba | FR |
| | geranyl acetate | FL/FR |
| (E)- | geranyl acetone | FL/FR |
| | geranyl formate | FL/FR |
| | geranyl hexanoate | FL/FR |
| (E)- | geranyl linalool | FL/FR |
| | globulol | |
| | hawthorn ethanol | FR |
| | heather fragrance | FR |
| | heliotrope absolute | FR |
| | herbal pyran | FR |
| (Z)-3- | hexen-1-yl salicylate | FL/FR |
| alpha- | hexyl cinnamaldehyde | FL/FR |
| | hexyl oxyacetonitrile | |
| | ho leaf oil | FR |
| | ho wood oil | FR |
| | hydroxycitronellal | FL/FR |
| | hydroxycitronellal dimethyl acetal | FL/FR |
| | hydroxycitronellol | FL/FR |
| | ilex paraguariensis oleoresin | FL/FR |
| beta- | ionol | FL/FR |
| beta- | ionone | FL/FR |
| (Z)- | jasmone | FL/FR |
| iso | jasmone | FL/FR |
| | karo karounde absolute | FR |
| | lavandin concrete decolorized | FR |
| | lavandin oil | FL/FR |
| | lavandula angustifolia flower oil | FL/FR |
| | lavender absolute replacer | FR |
| | lavender oil | FL/FR |
| | lavender oil bulgaria | FL/FR |
| | lavender oil france | FL/FR |
| | lavender oil greece | FL/FR |
| | lavender oil replacer | FR |
| laevo- | linalool | FL/FR |
| dextro- | linalool | FL/FR |
| | linalool | FL/FR |
| | linalool oxide | FL/FR |
| | linalyl anthranilate | FL/FR |
| | methyl nerate | |
| 2- | methyl octanal | FL/FR |
| | mimosa absolute morocco | FL/FR |
| | muguet butanal | FR |
| | musk acetate | FR |
| | neroli oil CO2 extract | FL/FR |
| | nerolidol | FL/FR |
| (E)- | nerolidol | FL/FR |
| | neryl acetate | FL/FR |
| | neryl isovalerate | FL/FR |
| | nonanol | FL/FR |
| | nonisyl propionate | FR |
| (Z)-beta- | ocimene | FL/FR |
| | octyl acetate | FL/FR |
| | orange leaf absolute | FL/FR |
| | papaya isobutyrate | FL/FR |
| | pelargonium graveolens flower water | FR |
| | petitgrain lemon oil | FL/FR |
| | phenethyl acetate | FL/FR |
| | phenethyl anthranilate | FL/FR |
| | phenethyl butyrate | FL/FR |
| | phenethyl phenyl acetate | FL/FR |
| | phenethyl salicylate | FL/FR |
| | phenyl acetaldehyde digeranyl acetal | FR |
| 2- | phenyl propionaldehyde dimethyl acetal | FL/FR |
| iso | phytol | FL/FR |
| | prenyl salicylate | FL/FR |
| | rhodinol | FL/FR |
| | rose absolute (rosa damascena) bulgaria | FL/FR |
| | rose butanoate | FL/FR |
| laevo- | rose oxide | FL/FR |
| | sambucus nigra flower oil CO2 extract | FR |
| | styralyl propionate | FL/FR |
| | terpinyl isobutyrate | FL/FR |
| | tetrahydrolinalool | FL/FR |
| | tetrahydrolinalyl acetate | FR |
| 2- | undecen-1-ol | FL/FR |
| (E)-2- | undecen-1-ol | FL/FR |
| fruity |
| | acetaldehyde dihexyl acetal | FL/FR |
| iso | butyl citral | |
| (E)-alpha- | damascone | FL/FR |
| | decen-1-yl cyclopentanone | FL/FR |
| | decyl butyrate | FL/FR |
| | geranyl butyrate | FL/FR |
| | methyl acetoacetate | FL/FR |
| | methyl anthranilate | FL/FR |
| (E)-2- | nonen-1-yl acetate | FL/FR |
| | prenyl acetate | FL/FR |
| | sambucus canadensis fruit absolute | FL/FR |
| | strawberry glycidate 2 | FL/FR |
| (E)-2- | undecenal | FL/FR |
| green |
| | acetaldehyde di-(Z)-3-hexen-1-yl acetal | FL/FR |
| | acetaldehyde ethyl phenethyl acetal | FL/FR |
| alpha- | allyl-4-methyl-3-cyclohexene-1-methanyl acetate | |
| iso | butyl benzyl carbinol | FL/FR |
| iso | decanal | FL/FR |
| 2,6- | dimethyl octanal | FL/FR |
| (Z)-beta- | farnesene | |
| | green carbaldehyde | FR |
| | green carboxylate | FR |
| | heptyl formate | FL/FR |
| 2- | heptyl tetrahydrofuran | FR |
| (Z)-3- | hexen-1-yl angelate | FR |
| (Z)-3- | hexen-1-yl benzoate | FL/FR |
| (E)-2- | hexen-1-yl hexanoate | FL/FR |
| (Z)-3- | hexen-1-yl oxyacetaldehyde | FR |
| (Z)-3- | hexen-1-yl phenyl acetate | FL/FR |
| | marigold pot absolute | FL/FR |
| | octanal dimethyl acetal | FL/FR |
| | phenethyl tiglate | FL/FR |
| | phenyl acetaldehyde | FL/FR |
| | phenyl acetaldehyde dimethyl acetal | FL/FR |
| herbal |
| | calendula officinalis flower oil CO2 extract | FR |
| | cananga fruit oil | FR |
| | carrot seed oil (daucus carota ssp. gummifer hook. fil.) spain | FR |
| | chamomile isobutyrate | FR |
| | chrysanthemum ketone | FR |
| | coriander seed absolute | FL/FR |
| beta- | elemene | FL/FR |
| | inula root oil | FR |
| | lavandin flower water | FR |
| | lavender absolute bulgaria | FL/FR |
| | lavender oil terpeneless | FL/FR |
| | lavender stem oil lithuania | FR |
| | linalyl acetate | FL/FR |
| | linalyl formate | FL/FR |
| | marigold pot flower oil | FL/FR |
| laevo- | menthyl propionate | FL/FR |
| | methyl cyclogeranate (Firmenich) | FR |
| | methyl ortho-anisate | FL/FR |
| | myrtle oil morocco | FL/FR |
| | nonisyl acetate | FR |
| 3- | octyl acetate | FL/FR |
| | rosmarinus officinalis extract | FL/FR |
| alpha- | terpinyl acetate | FL/FR |
| 2,4,6- | trimethyl-6-(3-methyl-2-buten-1-yl)-2-cyclohexen-1-one | |
| honey |
| | phenyl acetic acid | FL/FR |
| spicy |
| alpha- | amyl cinnamyl alcohol | FL/FR |
| | bayberry fragrance | FR |
| | carnation absolute | FR |
| | cubeb oil | FL/FR |
| | pimenta acris leaf oil | FL/FR |
| waxy |
| | aldenal C-9 | FR |
| | decanal dimethyl acetal | FL/FR |
| | ethyl nonanoate | FL/FR |
| | orris rhizome oil CO2 extract | FL/FR |
| (E)-2- | tridecenal | FL/FR |
| 1- | undecanol | FL/FR |
| (E)-2- | undecen-1-yl acetate | FR |
| | waxy acetate | FR |
| woody |
| | sandal cyclopentane | FR |
| alpha- | terpinene | FL/FR |
| | zdravetz absolute | FR |
| | zdravetz oil | FL/FR |
| |
| For Flavor |
| |
| No flavor group found for these |
| | acetaldehyde di-(Z)-3-hexen-1-yl acetal | FL/FR |
| ortho- | acetyl-para-cresol | FL/FR |
| iso | amyl undecylenate | FL/FR |
| | benzyl pivalate | FL/FR |
| | butyl benzyl ether | FL/FR |
| iso | butyl citral | |
| | cassie absolute replacer | FL/FR |
| | cyclohexyl benzoate | FL/FR |
| (Z)-alpha- | damascone | FL/FR |
| iso | decanal | FL/FR |
| | decanal dimethyl acetal | FL/FR |
| (Z)-4- | decen-1-yl acetate | FL/FR |
| 9- | decenal | FL/FR |
| (Z)-4- | dodecenal | FL/FR |
| beta- | elemene | FL/FR |
| | ethyl linalyl ether | FL/FR |
| (Z)-beta- | farnesene | |
| (E)- | geranyl linalool | FL/FR |
| | globulol | |
| | hexyl oxyacetonitrile | |
| | ilex paraguariensis oleoresin | FL/FR |
| | jambu flower oil brazil | |
| dextro- | linalool | FL/FR |
| (Z)- | linalool oxide (pyranoid) | FL/FR |
| | marigold pot flower oil | FL/FR |
| laevo- | menthyl propionate | FL/FR |
| | methyl acetoacetate | FL/FR |
| | methyl nerate | |
| 2- | methyl octanal | FL/FR |
| | methyl ortho-anisate | FL/FR |
| | myrtle oil morocco | FL/FR |
| | nonanal diethyl acetal | FL/FR |
| | octyl phenyl acetate | FL/FR |
| | terpinyl isobutyrate | FL/FR |
| | tetrahydrocitral | FL/FR |
| 2,4,6- | trimethyl-6-(3-methyl-2-buten-1-yl)-2-cyclohexen-1-one | |
| 2- | undecen-1-ol | FL/FR |
| (E)-2- | undecen-1-ol | FL/FR |
| (E)-4- | undecenal | FL |
| | zdravetz oil | FL/FR |
| aldehydic |
| | acetyl nonyryl | FL/FR |
| | nonanal (aldehyde C-9) | FL/FR |
| | octanal (aldehyde C-8) | FL/FR |
| 2- | tridecenal | FL/FR |
| 1- | undecanol | FL/FR |
| amber |
| iso | butyl benzyl carbinol | FL/FR |
| aromatic |
| | amyl salicylate | FL/FR |
| balsamic |
| | benzyl benzoate | FL/FR |
| | benzyl salicylate | FL/FR |
| citrus |
| | bergamot oil bergaptene reduced italy | FL/FR |
| | bergamot oil turkey | FL/FR |
| beta- | bisabolol | FL/FR |
| | citral diethyl acetal | FL/FR |
| | citral dimethyl acetal | FL/FR |
| | citronellyl oxyacetaldehyde | FL/FR |
| | citrus limon peel oil expressed | FL/FR |
| | citrus paradisi peel extract | FL/FR |
| (Z)-4- | decenal | FL/FR |
| | lemon oil c.p. california | FL/FR |
| | lemon oil c.p. furocoumarin reduced | FL/FR |
| | lemongrass oil | FL/FR |
| | linalool | FL/FR |
| laevo- | linalool | FL/FR |
| | orange fruit oil | FL/FR |
| blood | orange oil italy | FL/FR |
| | petitgrain lemon oil | FL/FR |
| | styralyl propionate | FL/FR |
| | verbena absolute france | FL/FR |
| | verbena oil terpeneless | FL/FR |
| fatty |
| 2,4- | decadien-1-ol | FL/FR |
| | dimethyl octanol | FL/FR |
| | heptyl formate | FL/FR |
| (Z)-3- | hexen-1-yl benzoate | FL/FR |
| 10- | undecenal (aldehyde C-11 undecylenic) | FL/FR |
| floral |
| | bois de rose oil brazil | FL/FR |
| | citronellol | FL/FR |
| (S)- | citronellyl acetate | FL/FR |
| | citronellyl phenyl acetate | FL/FR |
| | citronellyl propionate | FL/FR |
| | dihydrocarvyl acetate | FL/FR |
| | dihydrojasmone | FL/FR |
| | dihydrolinalool | FL/FR |
| | dimethyl benzyl carbinyl butyrate | FL/FR |
| | farnesyl acetate | FL/FR |
| | geraniol | FL/FR |
| (E)- | geranyl acetone | FL/FR |
| beta- | ionol | FL/FR |
| | lepidine | FL/FR |
| | linalyl acetate | FL/FR |
| | linalyl anthranilate | FL/FR |
| | mimosa absolute morocco | FL/FR |
| | neroli oil CO2 extract | FL/FR |
| | neryl acetate | FL/FR |
| | orange leaf absolute | FL/FR |
| | phenethyl anthranilate | FL/FR |
| | phenyl acetic acid | FL/FR |
| | rhodinol | FL/FR |
| | rose absolute (rosa damascena) bulgaria | FL/FR |
| laevo- | rose oxide | FL/FR |
| | tetrahydrolinalool | FL/FR |
| fruity |
| | benzyl alcohol | FL/FR |
| | citronellyl butyrate | FL/FR |
| (E)-alpha- | damascone | FL/FR |
| | decen-1-yl cyclopentanone | FL/FR |
| | decyl butyrate | FL/FR |
| | geranyl butyrate | FL/FR |
| | geranyl hexanoate | FL/FR |
| | methyl anthranilate | FL/FR |
| | neryl isovalerate | FL/FR |
| | phenethyl butyrate | FL/FR |
| 2- | phenyl propionaldehyde dimethyl acetal | FL/FR |
| | prenyl acetate | FL/FR |
| | rose butanoate | FL/FR |
| | sambucus canadensis fruit absolute | FL/FR |
| | strawberry glycidate 2 | FL/FR |
| green |
| | acetaldehyde dihexyl acetal | FL/FR |
| | acetaldehyde ethyl phenethyl acetal | FL/FR |
| alpha- | allyl-4-methyl-3-cyclohexene-1-methanyl acetate | |
| iso | amyl salicylate | FL/FR |
| | cinnamyl alcohol | FL/FR |
| (E)-4- | decenal | FL/FR |
| 2,6- | dimethyl octanal | FL/FR |
| | gelsone (IFF) | FL/FR |
| | geranyl acetate | FL/FR |
| | geranyl formate | FL/FR |
| (E,E)-2,4- | hexadienal | FL |
| (E)-2- | hexen-1-yl hexanoate | FL/FR |
| (Z)-3- | hexen-1-yl phenyl acetate | FL/FR |
| (Z)-3- | hexen-1-yl salicylate | FL/FR |
| | hibiscus distillates | FL |
| iso | jasmone | FL/FR |
| | linalool oxide | FL/FR |
| | marigold pot absolute | FL/FR |
| | methyl heptenone | FL/FR |
| (E)- | nerolidol | FL/FR |
| | nerolidol | FL/FR |
| (E,E)-2,6- | nonadienal | FL |
| | nonanal dimethyl acetal | FL/FR |
| (E)-2- | nonenal | FL/FR |
| (Z)-beta- | ocimene | FL/FR |
| | octanal dimethyl acetal | FL/FR |
| 3- | octyl acetate | FL/FR |
| | papaya isobutyrate | FL/FR |
| | phenethyl tiglate | FL/FR |
| | phenyl acetaldehyde dimethyl acetal | FL/FR |
| herbal |
| | coriander seed absolute | FL/FR |
| 3,6- | dimethyl-3-octanol | FL/FR |
| | lavandin oil | FL/FR |
| | lavandula angustifolia flower oil | FL/FR |
| | lavender absolute bulgaria | FL/FR |
| | lavender oil | FL/FR |
| | lavender oil bulgaria | FL/FR |
| | lavender oil france | FL/FR |
| | lavender oil greece | FL/FR |
| | lavender oil terpeneless | FL/FR |
| | linalyl formate | FL/FR |
| | prenyl salicylate | FL/FR |
| | rosmarinus officinalis extract | FL/FR |
| honey |
| | phenethyl acetate | FL/FR |
| | phenethyl phenyl acetate | FL/FR |
| | phenyl acetaldehyde | FL/FR |
| medicinal, |
| | phenethyl salicylate | FL/FR |
| oily |
| iso | phytol | FL/FR |
| powdery |
| | hydroxycitronellol | FL/FR |
| soapy |
| | dodecanal (aldehyde C-12 lauric) | FL/FR |
| spicy |
| alpha- | amyl cinnamyl alcohol | FL/FR |
| | cubeb oil | FL/FR |
| | pimenta acris leaf oil | FL/FR |
| terpenic |
| alpha- | terpinene | FL/FR |
| waxy |
| | decanal (aldehyde C-10) | FL/FR |
| 2- | decen-1-ol | FL/FR |
| 9- | decen-1-ol | FL/FR |
| | ethyl nonanoate | FL/FR |
| alpha- | hexyl cinnamaldehyde | FL/FR |
| | hydroxycitronellal | FL/FR |
| | hydroxycitronellal dimethyl acetal | FL/FR |
| | methyl 10-undecenoate | FL/FR |
| | nonanol | FL/FR |
| (E)-2- | nonen-1-yl acetate | FL/FR |
| | octyl acetate | FL/FR |
| (E)-2- | tridecenal | FL/FR |
| | undecanal | FL/FR |
| (E)-2- | undecenal | FL/FR |
| woody |
| beta- | ionone | FL/FR |
| (Z)- | jasmone | FL/FR |
| | orris rhizome oil CO2 extract | FL/FR |
| alpha- | terpinyl acetate | FL/FR |
| |
Potential Uses:
Occurrence (nature, food, other): noteSynonyms:
| (+)- | citronellal | | (±)- | citronellal | | | citronellal (natural) | | | citronellal 80 | | | citronellal 85/90% FCC (natural) | | | citronellal china, natural isolated constituent | | | citronellal ex eucalyptus citriodora 85/90% | | | citronellal ex-citronella oil | | | citronellal ex-eucalyptus citriodara oil | | | citronellal extra | | | citronellal FCC natural | | | citronellal FCC, natural | | | citronellal FCC, synthetic | | | citronellal natural | | | citronellal natural FCC | | | citronellal naturel | | | citronellal synthetic | | 2,3- | dihydrocitral | | 3,7- | dimethyl-6-octen-1-al | | (±)-3,7- | dimethyl-6-octenal | | 3,7- | dimethyl-6-octenal | | 3,7- | dimethyloct-6-enal | | 6- | octenal, 3,7-dimethyl- | | | rhodinal | | D- | rhodinal | | dextro- | rhodinal |
Articles:
| PubMed: | Alkaline ionic liquids applied in supported ionic liquid catalyst for selective hydrogenation of citral to citronellal. |
| J-Stage: | Synthesis of All the Stereoisomers of 6-Methyl-2-octadecanone, 14-Methyl-2-octadecanone, and 6,14-Dimethyl-2-octadecanone, Sex Pheromone Components of the Lyclene dharma dharma Moth, from the Enantiomers of Citronellal |
| PubMed: | An isomer of citronellal. |
| J-Stage: | Citronellal Ingestion Decreases the Appeal of Male Mouse Urinary Pheromone for Female Mice |
| PubMed: | Dose-Dependent Behavioral Response of the Mosquito Aedes albopictus to Floral Odorous Compounds. |
| PubMed: | Enantioselectivity of the bioconversion of chiral citronellal during the inhibition of wheat seeds germination. |
| PubMed: | Fumigant antifungal activity of Corymbia citriodora and Cymbopogon nardus essential oils and citronellal against three fungal species. |
| PubMed: | Chemical composition and insecticidal activity of plant essential oils from Benin against Anopheles gambiae (Giles). |
| PubMed: | Chemical composition, cytotoxicity and in vitro antitrypanosomal and antiplasmodial activity of the essential oils of four Cymbopogon species from Benin. |
| PubMed: | Differential expression of olfactory genes in the southern house mosquito and insights into unique odorant receptor gene isoforms. |
| PubMed: | Biotransformations of terpenes by fungi from Amazonian citrus plants. |
| PubMed: | Use of olfactory training in post-traumatic and postinfectious olfactory dysfunction. |
| PubMed: | Synergistic interaction and mode of action of Citrus hystrix essential oil against bacteria causing periodontal diseases. |
| PubMed: | Formulas of components of citronella oil against mosquitoes (Aedes aegypti). |
| PubMed: | Enantioselective effects of (+)- and (-)-citronellal on animal and plant microtubules. |
| PubMed: | Herbivory by the insect diaphorina citri induces greater change in citrus plant volatile profile than does infection by the bacterium, Candidatus Liberibacter asiaticus. |
| PubMed: | Molluscicidal and larvicidal activities and essential oil composition of Cymbopogon winterianus. |
| PubMed: | Chemical composition and antimicrobial activity of Cymbopogon nardus citronella essential oil against systemic bacteria of aquatic animals. |
| PubMed: | Citronellal, a monoterpene present in Java citronella oil, attenuates mechanical nociception response in mice. |
| PubMed: | In vitro and in vivo anti-tobacco mosaic virus activities of essential oils and individual compounds. |
| PubMed: | Olfactory training in patients with Parkinson's disease. |
| PubMed: | Cytosolic monoterpene biosynthesis is supported by plastid-generated geranyl diphosphate substrate in transgenic tomato fruits. |
| PubMed: | Behavioural and electroantennogram responses of the stable fly (Stomoxys calcitrans L.) to plant essential oils and their mixtures with attractants. |
| PubMed: | Synthesis of palmyrolide A and its cis-isomer and mechanistic insight into trans-cis isomerisation of the enamide macrocycle. |
| PubMed: | Preliminary analysis of several attractants and spatial repellents for the mosquito, Aedes albopictus using an olfactometer. |
| PubMed: | A 3-D open-framework material with intrinsic chiral topology used as a stationary phase in gas chromatography. |
| PubMed: | Essential oils and their compositions as spatial repellents for pestiferous social wasps. |
| PubMed: | Evaluation of the masking of dimethyl sulfide odors by citronellal, limonene and citral through the use of trained odor sensor mice. |
| PubMed: | Selective monoterpene-like cyclization reactions achieved by water exclusion from reactive intermediates in a supramolecular catalyst. |
| PubMed: | Phytochemical composition of Cymbopogon citratus and Eucalyptus citriodora essential oils and their anti-inflammatory and analgesic properties on Wistar rats. |
| PubMed: | Chiroptical sensing of citronellal: systematic development of a stereodynamic probe using the concept of isostericity. |
| PubMed: | Synthesis of all the stereoisomers of 6-methyl-2-octadecanone, 14-methyl-2-octadecanone, and 6,14-dimethyl-2-octadecanone, sex pheromone components of the Lyclene dharma dharma moth, from the enantiomers of citronellal. |
| PubMed: | Toxicity of Zanthoxylum piperitum and Zanthoxylum armatum oil constituents and related compounds to Stomoxys calcitrans (Diptera: Muscidae). |
| PubMed: | Citronellal ingestion decreases the appeal of male mouse urinary pheromone for female mice. |
| PubMed: | Functional characterization of SlscADH1, a fruit-ripening-associated short-chain alcohol dehydrogenase of tomato. |
| PubMed: | Influence of extraction methodologies on the analysis of five major volatile aromatic compounds of citronella grass (Cymbopogon nardus) and lemongrass (Cymbopogon citratus) grown in Thailand. |
| PubMed: | Comparative molecular docking analysis of essential oil constituents as elastase inhibitors. |
| PubMed: | Thymol nanospheres as an effective anti-bacterial agent. |
| PubMed: | Contact and fumigant toxicity of Cyperus rotundus steam distillate constituents and related compounds to insecticide-susceptible and -resistant Blattella germanica. |
| PubMed: | The synergistic effects of insecticidal essential oils and piperonyl butoxide on biotransformational enzyme activities in Aedes aegypti (Diptera: Culicidae). |
| PubMed: | Variation in the volatile oil composition of Eucalyptus citriodora produced by hydrodistillation and supercritical fluid extraction techniques. |
| PubMed: | Prokaryotic squalene-hopene cyclases can be converted to citronellal cyclases by single amino acid exchange. |
| PubMed: | Plant terpenoids: acute toxicities and effects on flight motor activity and wing beat frequency in the blow fly Phaenicia sericata. |
| PubMed: | A piperidine chiron for the Veratrum alkaloids. |
| PubMed: | Nature-inspired total synthesis of (-)-fusarisetin A. |
| PubMed: | MOFs as multifunctional catalysts: one-pot synthesis of menthol from citronellal over a bifunctional MIL-101 catalyst. |
| PubMed: | A dual catalyst system provides the shortest pathway for L-menthol synthesis. |
| PubMed: | In silico analysis of enzyme involved in enrichment of citronella oil. |
| PubMed: | Scientific basis for the therapeutic use of Cymbopogon citratus, stapf (Lemon grass). |
| PubMed: | Activation-independent cyclization of monoterpenoids. |
| PubMed: | Concise formal synthesis of (-)-7-deoxyloganin via N-heterocyclic carbene catalysed rearrangement of α,β-unsaturated enol esters. |
| PubMed: | Microencapsulation of citronella oil for mosquito-repellent application: formulation and in vitro permeation studies. |
| PubMed: | Characterization of biofumigated Ralstonia solanacearum cells using micro-Raman spectroscopy and electron microscopy. |
| PubMed: | Microwave-assisted partial hydrogenation of citral by using ionic liquid-coated porous glass catalysts. |
| PubMed: | Repellent constituents of essential oil of Cymbopogon distans aerial parts against two stored-product insects. |
| PubMed: | Chemical composition analysis of the essential oil of Melissa officinalis L. from Kurdistan, Iran by HS/SPME method and calculation of the biophysicochemical coefficients of the components. |
| PubMed: | Terpenoids inhibit Candida albicans growth by affecting membrane integrity and arrest of cell cycle. |
| PubMed: | Antibacterial activity of essential oils from Eucalyptus and of selected components against multidrug-resistant bacterial pathogens. |
| PubMed: | Antinociceptive action and redox properties of citronellal, an essential oil present in lemongrass. |
| PubMed: | Antifungal activity of Cymbopogon winterianus jowitt ex bor against Candida albicans. |
| PubMed: | Comparison of Eucalyptus cinerea essential oils produced by hydrodistillation and supercritical carbon dioxide extraction. |
| PubMed: | Aliphatic C-H to C-C conversion: synthesis of (-)-cameroonan-7α-ol. |
| PubMed: | Enhanced repellency of binary mixtures of Zanthoxylum armatum seed oil, vanillin, and their aerosols to mosquitoes under laboratory and field conditions. |
| PubMed: | Seasonal variation in the chemical composition and antimicrobial activity of volatile oils of three species of Leptospermum (Myrtaceae) grown in Brazil. |
| PubMed: | On the synergistic catalytic properties of bimetallic mesoporous materials containing aluminum and zirconium: the Prins cyclisation of citronellal. |
| PubMed: | Anti-inflammatory and redox-protective activities of citronellal. |
| PubMed: | Alteration of mouse urinary odor by ingestion of the xenobiotic monoterpene citronellal. |
| PubMed: | The effects of high hydrostatic pressure treatment on the flavor and color of grated ginger. |
| PubMed: | Towards preparative-scale, biocatalytic alkene reductions. |
| PubMed: | Drosophila TRPA1 channel is required to avoid the naturally occurring insect repellent citronellal. |
| PubMed: | Antinociceptive effects of citronellal in formalin-, capsaicin-, and glutamate-induced orofacial nociception in rodents and its action on nerve excitability. |
| PubMed: | Antinociceptive effect of citronellal in mice. |
| PubMed: | The cobalt way to angucyclinones: asymmetric total synthesis of the antibiotics (+)-rubiginone B2, (-)-tetrangomycin, and (-)-8-O-methyltetrangomycin. |
| PubMed: | Pyrrolidinyl-camphor derivatives as a new class of organocatalyst for direct asymmetric Michael addition of aldehydes and ketones to beta-nitroalkenes. |
| PubMed: | One-step microwave-assisted asymmetric cyclisation/hydrogenation of citronellal to menthols using supported nanoparticles on mesoporous materials. |
| PubMed: | Volatile organic components of fresh leaves as indicators of indigenous and cultivated citrus species in Taiwan. |
| PubMed: | Biological activity of essential oils from Aloysia polystachya and Aloysia citriodora (Verbenaceae) against the soybean pest Nezara viridula (Hemiptera: Pentatomidae). |
| PubMed: | Comparative anticonvulsant activities of the essential oils (EOs) from Cymbopogon winterianus Jowitt and Cymbopogon citratus (DC) Stapf. in mice. |
| PubMed: | A divergent approach to the diastereoselective synthesis of several ant-associated iridoids. |
| PubMed: | Bioactivity against Tribolium castaneum Herbst (Coleoptera: Tenebrionidae) of Cymbopogon citratus and Eucalyptus citriodora essential oils grown in Colombia. |
| PubMed: | High loading fragrance encapsulation based on a polymer-blend: preparation and release behavior. |
| PubMed: | Semiochemical-mediated oviposition avoidance by female house flies, Musca domestica, on animal feces colonized with harmful fungi. |
| PubMed: | Solution to spectroscopy challenge 14. Citronellal. |
| PubMed: | Synthesis, structural characterization and antiproliferative and toxic bio-activities of copper(II) and nickel(II) citronellal N4-ethylmorpholine thiosemicarbazonates. |
| PubMed: | Chemical composition of Eucalyptus spp. essential oils and their insecticidal effects on Lutzomyia longipalpis. |
| PubMed: | Citral and carvone chemotypes from the essential oils of Colombian Lippia alba (Mill.) N.E. Brown: composition, cytotoxicity and antifungal activity. |
| PubMed: | In vitro larvicidal activity of geraniol and citronellal against Contracaecum sp (Nematoda: Anisakidae). |
| PubMed: | Catalytic conjugate additions of geminal bis(sulfone)s: expanding the chemistry of sulfones as simple alkyl anion equivalents. |
| PubMed: | Laboratory evaluation of products to reduce settling of sweetpotato whitefly adults. |
| PubMed: | Repellent activity of essential oils: a review. |
| PubMed: | Characterization and antioxidant activity of essential oils from fresh and decaying leaves of Eucalyptus tereticornis. |
| PubMed: | Grape waste extract obtained by supercritical fluid extraction contains bioactive antioxidant molecules and induces antiproliferative effects in human colon adenocarcinoma cells. |
| PubMed: | Essential oil of Lindera neesiana fruit: chemical analysis and its potential use in topical applications. |
| PubMed: | Coupling reactions of catechins with natural aldehydes and allyl alcohols and radical scavenging activities of the triglyceride-soluble products. |
| PubMed: | Chemical compositions and antioxidant/antimicrobial activities of various samples prepared from Schinus terebinthifolius leaves cultivated in Egypt. |
| PubMed: | Reactivity in the confined spaces of zeolites: the interplay between spectroscopy and theory to develop structure-activity relationships for catalysis. |
| PubMed: | A tin-tungsten mixed oxide as an efficient heterogeneous catalyst for C-C bond-forming reactions. |
| PubMed: | Effects of olfactory training in patients with olfactory loss. |
| PubMed: | Chemical composition of the defensive secretion of the longhorned beetle, Chloridolum loochooanum. |
| PubMed: | Cyclisation of citronellal over heterogeneous inorganic fluorides--highly chemo- and diastereoselective catalysts for (+/-)-isopulegol. |
| PubMed: | Zr-zeolite beta: a new heterogeneous catalyst system for the highly selective cascade transformation of citral to (+/-)-menthol. |
| PubMed: | Larvicidal and oviposition-altering activity of monoterpenoids, trans-anithole and rosemary oil to the yellow fever mosquito Aedes aegypti (Diptera: Culicidae). |
| PubMed: | Synthesis of a dialuminum-substituted silicotungstate and the diastereoselective cyclization of citronellal derivatives. |
| PubMed: | Effects of chemopreventive citrus phytochemicals on human P-glycoprotein and multidrug resistance protein 1. |
| PubMed: | Activity of essential oils and individual components against acetyl- and butyrylcholinesterase. |
| PubMed: | The promoting effect of a dicyanamide based ionic liquid in the selective hydrogenation of citral. |
| PubMed: | Melissa officinalis oil affects infectivity of enveloped herpesviruses. |
| PubMed: | Feasibility of detection and quantification of gas-phase carbonyls in indoor environments using PFBHA derivatization and solid-phase microextraction (SPME). |
| PubMed: | Essential oil composition of three accessions of Dracocephalum heterophyllum Benth. cultivated at Palampur, India. |
| PubMed: | Oxidation of citronellal to citronellic acid by molecular oxygen using supported gold catalysts. |
| PubMed: | Chemical composition and in vitro antimicrobial activity of essential oil of Melissa officinalis L. from Romania. |
| PubMed: | Host-seeking and blood-feeding behavior of Aedes albopictus (Diptera: Culicidae) exposed to vapors of geraniol, citral, citronellal, eugenol, or anisaldehyde. |
| PubMed: | Citral derived amides as potent bacterial NorA efflux pump inhibitors. |
| PubMed: | Anti-inflammation activity of fruit essential oil from Cinnamomum insularimontanum Hayata. |
| PubMed: | Inhibitory effects of terpenoids on multidrug resistance-associated protein 2- and breast cancer resistance protein-mediated transport. |
| PubMed: | First asymmetric total syntheses of cernuane-type Lycopodium alkaloids, cernuine, and cermizine D. |
| PubMed: | Synthesis and catalysis of di- and tetranuclear metal sandwich-type silicotungstates [(gamma-SiW10O36)2M2(mu-OH)2]10- and [(gamma-SiW10O36)2M4(mu4-O)(mu-OH)6]8- (M = Zr or Hf). |
| PubMed: | Polar intermetallic compounds as catalysts for hydrogenation reactions: synthesis, structures, bonding, and catalytic properties of Ca(1-x)Sr(x)Ni4Sn2 (x=0.0, 0.5, 1.0) and catalytic properties of Ni3Sn and Ni3Sn2. |
| PubMed: | Efficient and selective synthesis of (S,R,R,S,R,S)-4,6,8,10,16,18-hexamethyl-docosane via Zr-catalyzed asymmetric carboalumination of alkenes (ZACA reaction). |
| PubMed: | Antimicrobial activity and potential use of monoterpenes as tropical fruits preservatives. |
| PubMed: | Expression of two old yellow enzyme homologues from Gluconobacter oxydans and identification of their citral hydrogenation abilities. |
| PubMed: | Phythochemical screening and anticonvulsant activity of Cymbopogon winterianus Jowitt (Poaceae) leaf essential oil in rodents. |
| PubMed: | Screening of antibacterial activities of twenty-one oxygenated monoterpenes. |
| PubMed: | Differences in (-)citronellal binding to various odorant receptors. |
| PubMed: | Structural determinants of odorant recognition by the human olfactory receptors OR1A1 and OR1A2. |
| PubMed: | Evaluation of phototoxic properties of fragrances. |
| PubMed: | Toxicity of Myristica fagrans seed compounds against Blattella germanica (Dictyoptera: Blattellidae). |
| PubMed: | Enantioselective total synthesis of isishippuric acid B via intramolecular Michael reaction. |
| PubMed: | High-performance liquid chromatographic method for the simultaneous determination of 24 fragrance allergens to study scented products. |
| PubMed: | Repellence of the red bud borer Resseliella oculiperda from grafted apple trees by impregnation of rubber budding strips with essential oils. |
| PubMed: | Antibiogram and GC analysis of Euphorbia hirta leaf extract. |
| PubMed: | Assessment of sensitization potential of monoterpenes using the rat popliteal lymph node assay. |
| PubMed: | Comparison of three lychee cultivar odor profiles using gas chromatography-olfactometry and gas chromatography-sulfur detection. |
| PubMed: | Authenticity control of essential oils containing citronellal and citral by chiral and stable-isotope gas-chromatographic analysis. |
| PubMed: | First enantiospecific synthesis of (-)-parvifoline and (-)-curcuquinone. |
| PubMed: | Antimicrobial effect of trans-cinnamaldehyde, (-)-perillaldehyde, (-)-citronellal, citral, eugenol and carvacrol on airborne microbes using an airwasher. |
| PubMed: | Chemical composition and phytotoxicity of volatile essential oil from intact and fallen leaves of Eucalyptus citriodora. |
| PubMed: | Inhibition of P-glycoprotein-mediated transport by terpenoids contained in herbal medicines and natural products. |
| PubMed: | Probing the Lewis acidity and catalytic activity of the metal-organic framework [Cu3(btc)2] (BTC=benzene-1,3,5-tricarboxylate). |
| PubMed: | Phytotoxicity of the volatile monoterpene citronellal against some weeds. |
| PubMed: | Foot odor due to microbial metabolism and its control. |
| PubMed: | Enthalpy change on mixing a couple of S- and R-enantiomers of some chiral compounds at 298.15 K. |
| PubMed: | Lipolytic effects of citrus peel oils and their components. |
| PubMed: | Chemical composition and inhibitory activity of essential oil from decaying leaves of Eucalyptus citriodora. |
| PubMed: | Acaricidal activities of paeonol and benzoic acid from Paeonia suffruticosa root bark and monoterpenoids against Tyrophagus putrescentiae (Acari: Acaridae). |
| PubMed: | [Extraction and determination of volatile constituents in leaves of Eucalyptus citriodora]. |
| PubMed: | Domino-cyclisation and hydrogenation of citronellal to menthol over bifunctional Ni/Zr-Beta and Zr-beta/Ni-MCM-41 catalysts. |
| PubMed: | Effects of citronellal, a monoterpenoid in Zanthoxyli Fructus, on the intestinal absorption of digoxin in vitro and in vivo. |
| PubMed: | Insecticidal activity of selected monoterpenoids and rosemary oil to Agriotes obscurus (Coleoptera: Elateridae). |
| PubMed: | Volatile constituents of redblush grapefruit (Citrus paradisi) and pummelo (Citrus grandis) peel essential oils from Kenya. |
| PubMed: | A highly selective, organocatalytic route to chiral dihydro-1,2-oxazines. |
| PubMed: | Photochemical release of aldehydes from alpha-acetoxy nitroveratryl ethers. |
| PubMed: | Pectin gel vehicles for controlled fragrance delivery. |
| PubMed: | Investigation of factors affecting the adsorption of functional molecules onto gel silicas. 1. Flow microcalorimetry and infrared spectroscopy. |
| PubMed: | Type 4 phosphodiesterase inhibition impairs detection of low odor concentrations in mice. |
| PubMed: | Inhibition of P-glycoprotein-mediated transport by extracts of and monoterpenoids contained in Zanthoxyli fructus. |
| PubMed: | Identification of specific ligands for orphan olfactory receptors. G protein-dependent agonism and antagonism of odorants. |
| PubMed: | Repellency of essential oils of some Kenyan plants against Anopheles gambiae. |
| PubMed: | Micelle-hosted palladium nanoparticles catalyze citral molecule hydrogenation in supercritical carbon dioxide. |
| PubMed: | Synthesis of the four stereoisomers of 7-acetoxy-15-methylnonacosane, a component of the female sex pheromone of the screwworm fly, Cochliomyia hominivorax. |
| PubMed: | Differences in the volatile components and their odor characteristics of green and ripe fruits and dried pericarp of Japanese pepper (Xanthoxylum piperitum DC.). |
| PubMed: | Ir-Beta zeolite as a heterogeneous catalyst for the one-pot transformation of citronellal to menthol. |
| PubMed: | Antimicrobial and antioxidant activities of Melissa officinalis L. (Lamiaceae) essential oil. |
| PubMed: | Utilisation of on-line acoustic irradiation as a means to counter-effect catalyst deactivation in heterogeneous catalysis. |
| PubMed: | Variability in the content and composition of essential oil from lemon balm (Melissa officinalis L.) cultivated in Poland. |
| PubMed: | Sn-Beta zeolite as diastereoselective water-resistant heterogeneous Lewis-acid catalyst for carbon-carbon bond formation in the intramolecular carbonyl-ene reaction. |
| PubMed: | Synthesis of the enantiomers of 21-methyl-7-hentriacontanone and a stereoisomeric mixture of 5-acetoxy-19-methylnonacosane, candidates for the female sex pheromone of the screwworm fly Cochliomyia hominivorax. |
| PubMed: | Receptor contributions to configural and elemental odor mixture perception. |
| PubMed: | Biotransformation of citronellal by Solanum aviculare suspension cultures: preparation of p-menthane-3,8-diols and determination of their absolute configurations. |
| PubMed: | Assessing the separation of neutral plant secondary metabolites by micellar electrokinetic chromatography. |
| PubMed: | Identification of aroma active compounds in orange essence oil using gas chromatography-olfactometry and gas chromatography-mass spectrometry. |
| PubMed: | Catalyst-controlled inverse-electron-demand hetero-Diels-Alder reactions in the enantio- and diastereoselective synthesis of iridoid natural products. |
| PubMed: | Character impact odorants of Citrus Hallabong [(C. unshiu Marcov x C. sinensis Osbeck) x C. reticulata Blanco] cold-pressed peel oil. |
| PubMed: | Inhibition of citrus postharvest pathogens by vapor of citral and related compounds in culture. |
| PubMed: | Synthesis of the four stereoisomers of 3,12-dimethylheptacosane, (Z)-9-pentacosene and (Z)-9-heptacosene, the cuticular hydrocarbons of the ant, Diacamma sp.. |
| PubMed: | Improved hardware and software for quick gas chromatography-olfactometry using CHARM and GC-"SNIF" analysis. |
| PubMed: | Effects of storage conditions on the composition of Citrus tamurana Hort. ex Tanaka (hyuganatsu) essential oil. |
| PubMed: | Characteristic odor components of Citrus sphaerocarpa Tanaka (Kabosu) cold-pressed peel oil. |
| PubMed: | Susceptibility of the bruchid Callosobruchus maculatus (Coleoptera: Bruchidae) and its parasitoid Dinarmus basalis (Hymenoptera: Pteromalidae) to three essential oils. |
| PubMed: | Comparison of extraction methods for marker compounds in the essential oil of lemon grass by GC. |
| PubMed: | The influence of the harvest cut height on the quality of the herbal drugs Melissae folium and Melissae herba. |
| PubMed: | Volatile components of peel and leaf oils of lemon and lime species. |
| PubMed: | Isolation of a deet-insensitive mutant of Drosophila melanogaster (Diptera: Drosophilidae). |
| PubMed: | New Lewis-Acidic Molybdenum(II) and Tungsten(II) Catalysts for Intramolecular Carbonyl Ene and Prins Reactions. Reversal of the Stereoselectivity of Cyclization of Citronellal. |
| PubMed: | Evaluation of vetiver oil and seven insect-active essential oils against the Formosan subterranean termite. |
| PubMed: | Local anaesthetic activity of monoterpenes and phenylpropanes of essential oils. |
| PubMed: | Synthesis and absolute configuration of Stellettadine A: a marine alkaloid that induces larval metamorphosis in ascidians. |
| PubMed: | A threshold-like measure for the assessment of olfactory sensitivity: the "random" procedure. |
| PubMed: | Changes in essential oil during enzyme-assisted ensiling of lemongrass (Cymbopogon citratus Stapf.) and lemon eucalyptus (Eucalyptus citriodora Hook). |
| PubMed: | Specific structural determinants are responsible for the antioxidant activity and the cell cycle effects of resveratrol. |
| PubMed: | Acute, sublethal, antifeedant, and synergistic effects of monoterpenoid essential oil compounds on the tobacco cutworm, Spodoptera litura (Lep., Noctuidae). |
| PubMed: | Biotransformation of constituents of essential oils by germinating wheat seed. |
| PubMed: | The use of linear expressions of solute boiling point versus retention to indicate special interactions with the molecular rings of modified cyclodextrin phases in gas chromatography |
| PubMed: | Effects of the sniffing port air makeup in gas chromatography-olfactometry. |
| PubMed: | Effects of branched cyclodextrins on the solubility and stability of terpenes. |
| PubMed: | In vitro evaluation of the sensitization potential of weak contact allergens using langerhans-like dendritic cells and autologous T cells. |
| PubMed: | Solid phase microextraction of volatile constituents from individual fresh Eucalyptus leaves of three species. |
| PubMed: | Ethnobotany, volatile oils and secretion tissues of werneria poposa from Argentina. |
| PubMed: | Odor suppression of voltage-gated currents contributes to the odor-induced response in olfactory neurons. |
| PubMed: | Grapefruit gland oil composition is affected by wax application, storage temperature, and storage time. |
| PubMed: | Sex steroids and odorants modulate gonadotropin-releasing hormone secretion in primary cultures of human olfactory cells. |
| PubMed: | Potentiation of GABAA receptors expressed in Xenopus oocytes by perfume and phytoncid. |
| PubMed: | Early changes in murine epidermal cell phenotype by contact sensitizers. |
| PubMed: | Identification of ligands for olfactory receptors by functional expression of a receptor library. |
| PubMed: | Preparation, Characterization, and Catalytic Properties of Polymer-Stabilized Ruthenium Colloids. |
| PubMed: | The poultry red mite Dermanyssus gallinae (De Geer, 1778): current situation and future prospects for control. |
| PubMed: | [Study of the olfactory effects of citronellal in the environmental air]. |
| PubMed: | Comparative repellency of commercial formulations of deet, permethrin and citronellal against the mosquito Aedes aegypti, using a collagen membrane technique compared with human arm tests. |
| PubMed: | Mutagenicity testing (+/-)-camphor, 1,8-cineole, citral, citronellal, (-)-menthol and terpineol with the Salmonella/microsome assay. |
| PubMed: | Effect of urine-derived compounds on cAMP accumulation in mouse vomeronasal cells. |
| PubMed: | In vitro inhibition of CYP2B1 monooxygenase by beta-myrcene and other monoterpenoid compounds. |
| PubMed: | Coupling of Metabolism and Bioconversion: Microbial Esterification of Citronellol with Acetyl Coenzyme A Produced via Metabolism of Glucose in an Interface Bioreactor. |
| PubMed: | Decomposition of Terpenes by Ozone during Sampling on Tenax. |
| PubMed: | In vitro primary sensitization of hapten-specific T cells by cultured human epidermal Langerhans cells--a screening predictive assay for contact sensitizers. |
| PubMed: | The aphid sex pheromone cyclopentanoids: synthesis in the elucidation of structure and biosynthetic pathways. |
| PubMed: | Chemical ecology of astigmatid mites--XLV. (2R, 3R)-epoxyneral: sex pheromone of the acarid mite Caloglyphus sp. (Acarina: Acaridae). |
| PubMed: | Insecticidal properties of several monoterpenoids to the house fly (Diptera: Muscidae), red flour beetle (Coleoptera: Tenebrionidae), and southern corn rootworm (Coleoptera: Chrysomelidae). |
| PubMed: | Isolation and characterization of Pseudomonas aeruginosa mutants deficient in the utilization of the terpenoid citronellic acid. |
| PubMed: | Studies of new short-period method for delayed contact hypersensitivity assay in the guinea pig. (2). Studies of the enhancement effect of cyclophosphamide. |
| PubMed: | Vapour toxicity & repellence of some essential oils & terpenoids to adults of Aedes aegypti (L) (Diptera: Culicidae). |
| PubMed: | Studies of new short-period method for delayed contact hypersensitivity assay in the guinea pig. (I). Development and comparison with other methods. |
| PubMed: | Quantitative analysis on odor intensity and quality of optical isomers in turtle olfactory system. |
| PubMed: | Studies of the reductive biotransformation of selected carbonyl compounds by whole cells and extracts of baker's yeast, Saccharomyces carevisiae. |
| PubMed: | Catalysis by cytochrome P-450 of an oxidative reaction in xenobiotic aldehyde metabolism: deformylation with olefin formation. |
| PubMed: | Effect of selected chemicals on mosquito larval orientation behaviour using a new apparatus. |
| PubMed: | Olfactory sensitivity of two sympatric species of rice leaf folders (Lepidoptera: Pyralidae) to plant volatiles. |
| PubMed: | Cinnamyl derivatives and monoterpenoids as nonspecific ovipositional deterrents of the onion fly. |
| PubMed: | Value of the cutaneous basophil hypersensitivity (CBH) response for distinguishing weak contact sensitization from irritation reactions in the guinea pig. |
| PubMed: | Terpenoid biotransformation in mammals. V. Metabolism of (+)-citronellal, (+-)-7-hydroxycitronellal, citral, (-)-perillaldehyde, (-)-myrtenal, cuminaldehyde, thujone, and (+-)-carvone in rabbits. |
| PubMed: | Application of response surface methodology to evaluation of bioconversion experimental conditions. |
| PubMed: | [Chemical composition of the essential oil from melissa]. |
| PubMed: | A new one-step synthesis of hexahydrocannabinoid analogs. |
| PubMed: | Microbial transformation of terpenoids. I. Identification of metabolites produced by a pseudomonad from citronellal and citral. |
| PubMed: | Effect of various essential oils isolated from Douglas fir needles upon sheep and deer rumen microbial activity. |
| PubMed: | Chemotherapy of human carcinoma with citronellal and citral and their action on carcinoma tissue in its histological aspects up to healing. |
| PubMed: | [Clinical effect of d-Rhodinicacid and d-Citronellal on pulmonary tuberculosis]. |
| PubMed: | Experiments on the chemotherapeutic treatment of carcinoma with citral and citronellal and those combined with PCMB. |
| PubMed: | [Direct action of citral and citronellal on cancer cells]. |
|
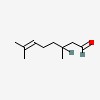 3D/inchi
3D/inchi






























