Articles:
phenylacetic acid
Notes:
associated with the inhibition of p21(ras) isoprenylation. Found in essential oils, e.g. neroli, rose oil, free and as esters. Also present in grapes, raspberry, strawberry, cherimoya, other fruits, cheddar cheese, Swiss cheese, wine, black tea, peated malt and other foodstuffs. Flavouring ingredient
Phenyl acetate (or phenylacetate) is a carboxylic acid ester that has been found in the biofluids of patients with nephritis and/or hepatitis as well as patients with phenylketonuria (PKU). Excess phenylalanine in the body can be disposed of through a transamination process leading to the production of phenylpyruvate. The phenylpyruvate can be further metabolized into a number of products. Decarboxylation of phenylpyruvate gives phenylacetate, while a reduction reaction gives phenyllactate. The phenylacetate can be further conjugated with glutamine to give phenylacetyl glutamine. All of these metabolites can be detected in serum and urine of PKU patients. Phenyl acetate is also produced endogenously as the metabolite of 2-Phenylethylamine, which is mainly metabolized by monoamine oxidase to form phenyl acetate. 2-phenylethylamine is an "endogenous amphetamine" which may modulate central adrenergic functions, and the urinary phenyl acetate levels have been postulated as a marker for depression. (PMID: 17978765, 476920, 6857245). Phenylacetate is also found in essential oils, e.g. neroli, rose oil, free and as esters' and in many fruits. As a result it is used as a perfumery and flavoring ingredient.; Phenylacetic acid (abr. PAA and synonyms are: ?-toluic acid, benzeneacetic acid, alpha tolylic acid, 2-phenylacetic acid) is an organic compound containing a phenyl functional group and an acetic acid functional group. It is a white solid with a disagreeable odor. Because it is used in the illicit production of phenylacetone (used in the manufacture of meth/amphetamines), it is subject to controls in the United States.
| Fragrance Demo Formulas Flavor Demo Formulas | ||
| CAS Number: | 103-82-2 | 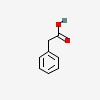 3D/inchi 3D/inchi
|
| ECHA EINECS - REACH Pre-Reg: | 203-148-6 | |
| FDA UNII: | ER5I1W795A | |
| Nikkaji Web: | J10.117F | |
| Beilstein Number: | 1099647 | |
| MDL: | MFCD00004313 | |
| CoE Number: | 672 | |
| XlogP3: | 1.40 (est) | |
| Molecular Weight: | 136.15016000 | |
| Formula: | C8 H8 O2 | |
| BioActivity Summary: | listing | |
| NMR Predictor: | Predict (works with chrome, Edge or firefox) | |
Category: flavor and fragrance agents
US / EU / FDA / JECFA / FEMA / FLAVIS / Scholar / Patent Information:
| Google Scholar: | Search |
| Google Books: | Search |
| Google Scholar: with word "volatile" | Search |
| Google Scholar: with word "flavor" | Search |
| Google Scholar: with word "odor" | Search |
| Perfumer and Flavorist: | Search |
| Google Patents: | Search |
| US Patents: | Search |
| EU Patents: | Search |
| Pubchem Patents: | Search |
| PubMed: | Search |
| NCBI: | Search |
| JECFA Food Flavoring: | 1007 phenylacetic acid |
| DG SANTE Food Flavourings: | 08.038 phenylacetic acid |
| FEMA Number: | 2878 phenylacetic acid |
| FDA: | No longer provide for the use of these seven synthetic flavoring substances |
| FDA Mainterm (SATF): | 103-82-2 ; PHENYLACETIC ACID |
| FDA Regulation: | |
| FDA PART 172 -- FOOD ADDITIVES PERMITTED FOR DIRECT ADDITION TO FOOD FOR HUMAN CONSUMPTION Subpart F--Flavoring Agents and Related Substances Sec. 172.515 Synthetic flavoring substances and adjuvants. | |
Physical Properties:
| Appearance: | white crystalline powder (est) |
| Assay: | 99.00 to 100.00 % |
| Heavey Metals: | <10.00 ppm |
| Food Chemicals Codex Listed: | Yes |
| Melting Point: | 76.00 to 78.00 °C. @ 760.00 mm Hg |
| Boiling Point: | 265.00 to 266.00 °C. @ 760.00 mm Hg |
| Boiling Point: | 142.00 to 143.00 °C. @ 10.00 mm Hg |
| Congealing Point: | 75.80 °C. |
| Vapor Pressure: | 0.005000 mmHg @ 25.00 °C. (est) |
| Vapor Density: | 4.0 ( Air = 1 ) |
| Flash Point: | 212.00 °F. TCC ( 100.00 °C. ) |
| logP (o/w): | 1.410 |
| Shelf Life: | 24.00 month(s) or longer if stored properly. |
| Storage: | store in cool, dry place in tightly sealed containers, protected from heat and light. |
| Soluble in: | |
| fixed oils | |
| glycerin | |
| water, 1.348e+004 mg/L @ 25 °C (est) | |
| Insoluble in: | |
| water | |
| Stability: | |
| bath foam | |
| cream | |
| hair spray | |
| lotion | |
| non-discoloring in most media | |
| powder | |
Organoleptic Properties:
| Odor Type: honey | |
| Odor Strength: | high , recommend smelling in a 10.00 % solution or less |
| Substantivity: | 400 hour(s) at 10.00 % in dipropylene glycol |
| sweet honey floral honeysuckle sour waxy civet | |
| Odor Description: at 10.00 % in dipropylene glycol. | sweet honey floral honeysuckle sour waxy civet Luebke, William tgsc, (1987) |
| sweet floral honey rose chocolate tobacco powdery animal | |
| Odor Description: | Sweet, floral, honey, rose, chocolate, tobacco and powdery with animal nuances Mosciano, Gerard P&F 21, No. 4, 51, (1996) |
| Flavor Type: floral | |
| sweet floral chocolate honey tobacco | |
| Taste Description: at 30.00 ppm. | Sweet, floral, chocolate, honey and tobacco Mosciano, Gerard P&F 21, No. 4, 51, (1996) |
| Odor and/or flavor descriptions from others (if found). | |
Cosmetic Information:
| CosIng: | cosmetic data |
| Cosmetic Uses: |
perfuming agents |
Suppliers:
Safety Information:
| Preferred SDS: View | |
| European information : | |
| Most important hazard(s): | |
| Xi - Irritant | |
|
R 36/37/38 - Irritating to eyes, respiratory system, and skin. S 02 - Keep out of the reach of children. S 24/25 - Avoid contact with skin and eyes. S 26 - In case of contact with eyes, rinse immediately with plenty of water and seek medical advice. S 37/39 - Wear suitable gloves and eye/face protection. | |
| Hazards identification | |
| Classification of the substance or mixture | |
| GHS Classification in accordance with 29 CFR 1910 (OSHA HCS) | |
| Skin irritation (Category 2), H315 Eye irritation (Category 2A), H319 Specific target organ toxicity - single exposure (Category 3), Respiratory system, H335 Reproductive toxicity (Category 2), H361 | |
| GHS Label elements, including precautionary statements | |
| Pictogram |  |
| Signal word | Warning |
| Hazard statement(s) | |
| H315 - Causes skin irritation H319 - Causes serious eye irritation H335 - May cause respiratory irritation H361 - Suspected of damaging fertility or the unborn child | |
| Precautionary statement(s) | |
| P201 - Obtain special instructions before use. P202 - Do not handle until all safety precautions have been read and understood. P261 - Avoid breathing dust/fume/gas/mist/vapours/spray. P264 - Wash skin thouroughly after handling. P271 - Use only outdoors or in a well-ventilated area. P280 - Wear protective gloves/protective clothing/eye protection/face protection. P302 + P352 - IF ON SKIN: wash with plenty of soap and water. P304 + P340 - IF INHALED: Remove victim to fresh air and Keep at rest in a position comfortable for breathing. P305 + P351 + P338 - IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continue rinsing. P308 + P313 - IF exposed or concerned: Get medical advice/attention. P332 + P313 - IF SKIN irritation occurs: Get medical advice/attention. P337 + P313 - IF eye irritation persists: Get medical advice/attention. P362 - Take off contaminated clothing and wash before reuse. P403 + P233 - Store in a well-ventilated place. Keep container tightly closed. P405 - Store locked up. P501 - Dispose of contents/ container to an approved waste disposal plant. | |
| Human Experience: | |
| 2 % solution: no irritation or sensitization. | |
| Oral/Parenteral Toxicity: | |
|
oral-rat LD50 > 5000 mg/kg (Keating, 1972c) gavage-guinea pig LD50 [sex: M,F] 2250 mg/kg (Zaitsev & Rakhmanina, 1974) gavage-mouse LD50 [sex: M,F] 2250 mg/kg (Zaitsev & Rakhmanina, 1974) gavage-rat LD50 [sex: M,F] 2250 mg/kg (Zaitsev & Rakhmanina, 1974) oral-guinea pig LD50 2250 mg/kg Voprosy Pitaniya. Problems of Nutrition. Vol. 33(5), Pg. 48, 1974. intraperitoneal-mouse LD50 2270 mg/kg Farmaco, Edizione Scientifica. Vol. 13, Pg. 286, 1958. oral-mouse LD50 2250 mg/kg Voprosy Pitaniya. Problems of Nutrition. Vol. 33(5), Pg. 48, 1974. intraperitoneal-rat LD50 1600 mg/kg Bollettino Chimico Farmaceutico. Vol. 112, Pg. 53, 1973. oral-rat LD50 2250 mg/kg Voprosy Pitaniya. Problems of Nutrition. Vol. 33(5), Pg. 48, 1974. | |
| Dermal Toxicity: | |
|
skin-rabbit LD50 > 5000 mg/kg Food and Cosmetics Toxicology. Vol. 13, Pg. 901, 1975. subcutaneous-mouse LD50 1500 mg/kg Archives Internationales de Pharmacodynamie et de Therapie. Vol. 116, Pg. 154, 1958. | |
| Inhalation Toxicity: | |
| Not determined | |
Safety in Use Information:
| Category: | flavor and fragrance agents | ||
| RIFM Fragrance Material Safety Assessment: Search | |||
| IFRA Code of Practice Notification of the 49th Amendment to the IFRA Code of Practice | |||
| Recommendation for phenyl acetic acid usage levels up to: | |||
| 2.0000 % in the fragrance concentrate. | |||
| Maximised Survey-derived Daily Intakes (MSDI-EU): | 240.00 (μg/capita/day) | ||
| Maximised Survey-derived Daily Intakes (MSDI-USA): | 60.00 (μg/capita/day) | ||
| Structure Class: | I | ||
| Use levels for FEMA GRAS flavoring substances on which the FEMA Expert Panel based its judgments that the substances are generally recognized as safe (GRAS). | |||
| The Expert Panel also publishes separate extensive reviews of scientific information on all FEMA GRAS flavoring substances and can be found at FEMA Flavor Ingredient Library | |||
| publication number: 3 | |||
| Click here to view publication 3 | |||
| average usual ppm | average maximum ppm | ||
| baked goods: | - | 12.00000 | |
| beverages(nonalcoholic): | - | 1.80000 | |
| beverages(alcoholic): | - | 0.10000 | |
| breakfast cereal: | - | - | |
| cheese: | - | - | |
| chewing gum: | 5.40000 | 11.00000 | |
| condiments / relishes: | - | - | |
| confectionery froastings: | - | - | |
| egg products: | - | - | |
| fats / oils: | - | - | |
| fish products: | - | - | |
| frozen dairy: | - | 5.30000 | |
| fruit ices: | - | 5.30000 | |
| gelatins / puddings: | - | 27.00000 | |
| granulated sugar: | - | - | |
| gravies: | - | - | |
| hard candy: | - | 5.90000 | |
| imitation dairy: | - | - | |
| instant coffee / tea: | - | - | |
| jams / jellies: | - | - | |
| meat products: | - | - | |
| milk products: | - | - | |
| nut products: | - | - | |
| other grains: | - | - | |
| poultry: | - | - | |
| processed fruits: | - | - | |
| processed vegetables: | - | - | |
| reconstituted vegetables: | - | - | |
| seasonings / flavors: | - | - | |
| snack foods: | - | - | |
| soft candy: | - | - | |
| soups: | - | - | |
| sugar substitutes: | - | - | |
| sweet sauces: | - | - | |
Safety References:
| Flavor & Extract Manufacturers Association (FEMA) reference(s): | |
| The FEMA GRAS assessment of phenethyl alcohol, aldehydes, acids, and related acetals and esters used as flavor ingredients. View pdf | |
| European Food Safety Athority(EFSA): | Flavor usage levels; Subacute, Subchronic, Chronic and Carcinogenicity Studies; Developmental / Reproductive Toxicity Studies; Genotoxicity Studies... |
| European Food Safety Authority (EFSA) reference(s): | |
| Flavouring Group Evaluation 55 (FGE.55): Consideration of phenyl-substituted aliphatic alcohols and related aldehydes and esters evaluated by JECFA (63rd meeting) structurally related to phenethyl alcohol, aldehyde, esters and related phenylacetic acid esters evaluated by EFSA in FGE.14 (2005) and aryl-substituted saturated and unsaturated primary alcohol/aldehyde/acid/ester derivatives evaluated by EFSA in FGE.15 (2005) (Commission Regulation (EC) No 1565/2000 of 18 July 2000) - Opinion of the Scientific Panel on Food Additives, Flavourings, Processing Aids and Materials in contact with Food (AFC) View page or View pdf | |
| Flavouring Group Evaluation 53 (FGE.53): Consideration of phenethyl alcohol, aldehyde, acid and related acetals and esters evaluated by JECFA (59th meeting) structurally related to phenethyl alcohol, aldehyde, esters and related phenylacetic acid esters evaluated by EFSA in FGE.14 (2005) and one phenoxyethyl ester evaluated in FGE.23 (2006) (Commission Regulation (EC) No 1565/2000 of 18 July 2000) - Opinion of the Scientific Panel on Food Additives, Flavourings, Processing Aids and Materials in contact with Food (AFC) View page or View pdf | |
| Flavouring Group Evaluation 33 (FGE.33)[1] - Six Tetrahydrofuran Derivatives from Chemical Groups 13, 14, 16 and 26 - Scientific Opinion of the Panel on Food Additives, Flavourings, Processing Aids and Materials in Contact with Food View page or View pdf | |
| Flavouring Group Evaluation 14, Revision 1 (FGE.14Rev1): Phenethyl alcohol, aldehyde, acetals, carboxylic acid and related esters from chemical group 15 and 22 [1] - Opinion of the Scientific Panel on Food Additives, Flavourings, Processing Aids and Materials in Contact with Food View page or View pdf | |
| Flavouring Group Evaluation 53, Revision 1 (FGE.53Rev1): Consideration of phenethyl alcohol, aldehyde, acid and related acetals and esters evaluated by JECFA (59th meeting) FGE.23Rev1 (2008) View page or View pdf | |
| EPI System: | View |
| ClinicalTrials.gov: | search |
| NIOSH International Chemical Safety Cards: | search |
| AIDS Citations: | Search |
| Cancer Citations: | Search |
| Toxicology Citations: | Search |
| EPA Substance Registry Services (TSCA): | 103-82-2 |
| EPA ACToR: | Toxicology Data |
| EPA Substance Registry Services (SRS): | Registry |
| Laboratory Chemical Safety Summary : | 999 |
| National Institute of Allergy and Infectious Diseases: | Data |
| WISER: | UN 3335 |
| WGK Germany: | 1 |
| 2-phenylacetic acid | |
| Chemidplus: | 0000103822 |
| RTECS: | 103-82-2 |
References:
| 2-phenylacetic acid | |
| NIST Chemistry WebBook: | Search Inchi |
| Canada Domestic Sub. List: | 103-82-2 |
| Pubchem (cid): | 999 |
| Pubchem (sid): | 134972411 |
| Flavornet: | 103-82-2 |
| Pherobase: | View |
Other Information:
| (IUPAC): | Atomic Weights of the Elements 2011 (pdf) |
| Videos: | The Periodic Table of Videos |
| tgsc: | Atomic Weights use for this web site |
| (IUPAC): | Periodic Table of the Elements |
| FDA Substances Added to Food (formerly EAFUS): | View |
| CHEBI: | View |
| CHEMBL: | View |
| Golm Metabolome Database: | Search |
| Metabolomics Database: | Search |
| UM BBD: | Search |
| KEGG (GenomeNet): | C07086 |
| HMDB (The Human Metabolome Database): | HMDB00209 |
| FooDB: | FDB010558 |
| YMDB (Yeast Metabolome Database): | YMDB00891 |
| Export Tariff Code: | 2916.34.1500 |
| Typical G.C. | |
| VCF-Online: | VCF Volatile Compounds in Food |
| ChemSpider: | View |
| Wikipedia: | View |
| Formulations/Preparations: grades: technical; fcc. | |
Potential Blenders and core components note
Potential Uses:
| absinthe | FL | |
| acacia | FR | |
| amber | FR | |
| animal | FR | |
| apple blossom | FR | |
| arnica flower | FR | |
| beeswax absolute replacer | FR | |
| bouquet | FR | |
| cappuccino | FL | |
| castoreum | FR | |
| chocolate cocoa | FL | |
| civet | FR | |
| clover | FR | |
| date | FR | |
| deertongue absolute replacer | FR | |
| elder berry | FR | |
| elder flower | FR | |
| fig | FR | |
| fixer | ||
| floral | FR | |
| genet | FR | |
| geranium | FR | |
| graham cracker | FR | |
| grape | FR | |
| heliotrope | FR | |
| honey | FR | |
| honeysuckle | FR | |
| immortelle | FL/FR | |
| jasmin | FR | |
| kewda | FR | |
| licorice | FR | |
| lilac | FR | |
| musk | FR | |
| narcissus | FR | |
| neroli | FR | |
| orange blossom | FR | |
| passion blossom | FR | |
| pumpkin pie | FR | |
| rose | FR | |
| rose red rose | FR | |
| sweet pea | FR | |
| tobacco | FR | |
| toffee | FR | |
| tropical | FL | |
| vanilla | FR | |
| violet | FR | |
| wallflower | FR |
Occurrence (nature, food, other): note
| almond flower Search Trop Picture | |
| cacao bean Search Trop Picture | |
| champaca concrete @ 0.20% Data GC Search Trop Picture | |
| cheese swiss cheese Search PMC Picture | |
| cichorium intybus l. root extract @ 2.49% Data GC Search Trop Picture | |
| cocoa bean Search Trop Picture | |
| corn pollen Search Trop Picture | |
| mango fruit Search Trop Picture | |
| neroli Search PMC Picture | |
| pea Search Trop Picture | |
| pepper black pepper fruit Search Trop Picture | |
| pepper black pepper oil Search Trop Picture | |
| pepper black pepper seed oil Search Trop Picture | |
| peppermint leaf Search Trop Picture | |
| rose bulgarian Search Trop Picture | |
| sake Search PMC Picture | |
| sherry Search PMC Picture | |
| tea leaf Search Trop Picture | |
| tobacco Search Trop Picture | |
| tomato Search Trop Picture | |
| walnut black walnut nut Search Trop Picture | |
| wine white wine Search Picture |
Synonyms:
| acetic acid, phenyl- | |
| benzenacetic acid | |
| benzene acetic acid | |
| benzeneacetic acid | |
| benzeneaceticacid | |
| benzeneacetiic acid | |
| benzyl carboxylic acid | |
| benzylcarboxylic acid | |
| benzylformic acid | |
| omega- | phenyl acetic acid |
| phenyl acetic acid natural | |
| phenyl acetic acid pure FCC | |
| phenyl ethanoic acid | |
| phenylacetic acid | |
| 2- | phenylacetic acid |
| phenylacetic acid natural | |
| phenylaceticacid | |
| 2- | phenylethanoic acid |
| phenyllacetic acid | |
| a- | toluic acid |
| alpha- | toluic acid |
| a- | tolylic acid |























