Articles:
4-hydroxybutyric acid lactone
Notes:
one of the furans with a carbonyl thereby forming a cyclic lactone. it is an endogenous compound made from gamma-aminobutyrate and is the precursor of gamma-hydroxybutyrate. it is also used as a pharmacological agent and solvent. Present in morello cherry, melon, pineapple, blackberry, quince, strawberry jam, wine, soybeans, black tea, Bourbon vanilla, wheat bread, crispbread and other breads. Flavour ingredient [DFC]
| Flavor Demo Formulas | ||
| CAS Number: | 96-48-0 | 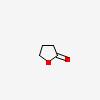 3D/inchi 3D/inchi
|
| Other(deleted CASRN): | 187997-16-6 | |
| ECHA EINECS - REACH Pre-Reg: | 202-509-5 | |
| FDA UNII: | OL659KIY4X | |
| Nikkaji Web: | J3.971C | |
| Beilstein Number: | 0105248 | |
| MDL: | MFCD00005386 | |
| CoE Number: | 615 | |
| XlogP3: | -0.60 (est) | |
| Molecular Weight: | 86.09022000 | |
| Formula: | C4 H6 O2 | |
| BioActivity Summary: | listing | |
| NMR Predictor: | Predict (works with chrome, Edge or firefox) | |
Category: flavor and fragrance agents
US / EU / FDA / JECFA / FEMA / FLAVIS / Scholar / Patent Information:
| Google Scholar: | Search |
| Google Books: | Search |
| Google Scholar: with word "volatile" | Search |
| Google Scholar: with word "flavor" | Search |
| Google Scholar: with word "odor" | Search |
| Perfumer and Flavorist: | Search |
| Google Patents: | Search |
| US Patents: | Search |
| EU Patents: | Search |
| Pubchem Patents: | Search |
| PubMed: | Search |
| NCBI: | Search |
| JECFA Food Flavoring: | 219 4-hydroxybutyric acid lactone |
| DG SANTE Food Flavourings: | 10.006 butyro-1,4-lactone |
| FEMA Number: | 3291 4-hydroxybutyric acid lactone |
| FDA: | No longer provide for the use of these seven synthetic flavoring substances |
| FDA Mainterm (SATF): | 96-48-0 ; 4-HYDROXYBUTANOIC ACID LACTONE |
Physical Properties:
| Appearance: | colorless to pale yellow clear liquid (est) |
| Assay: | 98.00 to 100.00 % |
| Food Chemicals Codex Listed: | Yes |
| Specific Gravity: | 1.12000 to 1.13000 @ 25.00 °C. |
| Pounds per Gallon - (est).: | 9.320 to 9.403 |
| Refractive Index: | 1.43000 to 1.44000 @ 20.00 °C. |
| Melting Point: | -43.30 °C. @ 760.00 mm Hg |
| Boiling Point: | 204.00 to 205.00 °C. @ 760.00 mm Hg |
| Vapor Pressure: | 0.450000 mmHg @ 25.00 °C. |
| Vapor Density: | 3.00 ( Air = 1 ) |
| Flash Point: | 209.00 °F. TCC ( 98.33 °C. ) |
| logP (o/w): | -0.640 |
| Soluble in: | |
| alcohol | |
| water, 1.00E+06 mg/L @ 25 °C (exp) | |
Organoleptic Properties:
| Odor Type: creamy | |
| creamy oily fatty caramellic | |
| Odor Description: at 100.00 %. | creamy oily fatty caramel |
| creamy oily fatty | |
| Odor Description: | Creamy, oily with fatty nuances Mosciano, Gerard P&F 16, No. 2, 49, (1991) |
| Flavor Type: milky | |
| milky creamy fruity peach | |
| Taste Description: at 75.00 ppm. | Milky, creamy with fruity peach-like afternotes Mosciano, Gerard P&F 16, No. 2, 49, (1991) |
| Odor and/or flavor descriptions from others (if found). | |
| Ernesto Ventós | |
| BUTYROLACTONE GAMMA | |
| Odor Description: | SWEET, AROMATIC, CREAMY |
| Indukern F&F | |
| GAMMA-BUTYROLACTONE | |
| Odor Description: | OILY, CREAMY, FATTY |
| Advanced Biotech | |
| GAMMA-BUTYROLACTONE SYNTHETIC | |
| Odor Description: | Caramel, Fatty, Creamy |
| Prodasynth | |
| GAMMA BUTYROLACTONE (> 98%) | |
| Odor Description: | SWEET, AROMATIC, CREAMY |
Cosmetic Information:
| CosIng: | cosmetic data |
| Cosmetic Uses: |
fragrance solvents |
Suppliers:
Safety Information:
| Preferred SDS: View | |
| European information : | |
| Most important hazard(s): | |
| Xn - Harmful. | |
|
R 22 - Harmful if swallowed. R 36 - Irritating to eyes. R 41 - Risk of serious damage to eyes. S 02 - Keep out of the reach of children. S 26 - In case of contact with eyes, rinse immediately with plenty of water and seek medical advice. S 36/39 - Wear suitable clothing and eye/face protection. | |
| Hazards identification | |
| Classification of the substance or mixture | |
| GHS Classification in accordance with 29 CFR 1910 (OSHA HCS) | |
| None found. | |
| GHS Label elements, including precautionary statements | |
| Pictogram | |
| Hazard statement(s) | |
| None found. | |
| Precautionary statement(s) | |
| None found. | |
| Oral/Parenteral Toxicity: | |
|
oral-rat LD50 1540 mg/kg LUNGS, THORAX, OR RESPIRATION: RESPIRATORY DEPRESSION BEHAVIORAL: SOMNOLENCE (GENERAL DEPRESSED ACTIVITY) BEHAVIORAL: ALTERED SLEEP TIME (INCLUDING CHANGE IN RIGHTING REFLEX) Gigiena Truda i Professional'nye Zabolevaniya. Labor Hygiene and Occupational Diseases. Vol. 31(1), Pg. 49, 1987. gavage-mouse LD50 1245 mg/kg (Schafer & Bowles, 1985) intraperitoneal-rat LD50 1000 mg/kg BEHAVIORAL: GENERAL ANESTHETIC LUNGS, THORAX, OR RESPIRATION: OTHER CHANGES Archivum Immunologiae et Therapiae Experimentalis. Vol. 13, Pg. 70, 1965. oral-mouse LD50 1460 mg/kg LUNGS, THORAX, OR RESPIRATION: RESPIRATORY DEPRESSION BEHAVIORAL: SOMNOLENCE (GENERAL DEPRESSED ACTIVITY) BEHAVIORAL: GENERAL ANESTHETIC Gigiena i Sanitariya. For English translation, see HYSAAV. Vol. 64(2), Pg. 3, 1999. intraperitoneal-mouse LD50 1100 mg/kg BEHAVIORAL: GENERAL ANESTHETIC LUNGS, THORAX, OR RESPIRATION: OTHER CHANGES Archivum Immunologiae et Therapiae Experimentalis. Vol. 13, Pg. 70, 1965. parenteral-mouse LDLo 1600 mg/kg "Summary Tables of Biological Tests," National Research Council Chemical-Biological Coordination Center. Vol. 7, Pg. 687, 1955. intravenous-rabbit LDLo 500 mg/kg Archivum Immunologiae et Therapiae Experimentalis. Vol. 13, Pg. 70, 1965. | |
| Dermal Toxicity: | |
|
skin-guinea pig LD50 > 5000 mg/kg GAF Material Safety Data Sheet. | |
| Inhalation Toxicity: | |
|
inhalation-rat LC50 > 5100 mg/m3/4H National Technical Information Service. Vol. OTS0534527 | |
Safety in Use Information:
| Category: | flavor and fragrance agents | ||
| RIFM Fragrance Material Safety Assessment: Search | |||
| IFRA Code of Practice Notification of the 49th Amendment to the IFRA Code of Practice | |||
| Recommendation for gamma-butyrolactone usage levels up to: | |||
| 0.5000 % in the fragrance concentrate. | |||
| Maximised Survey-derived Daily Intakes (MSDI-EU): | 110.00 (μg/capita/day) | ||
| Use levels for FEMA GRAS flavoring substances on which the FEMA Expert Panel based its judgments that the substances are generally recognized as safe (GRAS). | |||
| The Expert Panel also publishes separate extensive reviews of scientific information on all FEMA GRAS flavoring substances and can be found at FEMA Flavor Ingredient Library | |||
| publication number: 5 | |||
| Click here to view publication 5 | |||
| average usual ppm | average maximum ppm | ||
| baked goods: | - | 20.00000 | |
| beverages(nonalcoholic): | - | 10.00000 | |
| beverages(alcoholic): | - | - | |
| breakfast cereal: | - | 20.00000 | |
| cheese: | - | - | |
| chewing gum: | - | - | |
| condiments / relishes: | - | - | |
| confectionery froastings: | - | - | |
| egg products: | - | - | |
| fats / oils: | - | - | |
| fish products: | - | - | |
| frozen dairy: | - | 10.00000 | |
| fruit ices: | - | 10.00000 | |
| gelatins / puddings: | - | - | |
| granulated sugar: | - | - | |
| gravies: | - | 10.00000 | |
| hard candy: | - | 10.00000 | |
| imitation dairy: | - | - | |
| instant coffee / tea: | - | - | |
| jams / jellies: | - | - | |
| meat products: | - | 10.00000 | |
| milk products: | - | 10.00000 | |
| nut products: | - | - | |
| other grains: | - | - | |
| poultry: | - | - | |
| processed fruits: | - | - | |
| processed vegetables: | - | - | |
| reconstituted vegetables: | - | - | |
| seasonings / flavors: | - | - | |
| snack foods: | - | - | |
| soft candy: | - | - | |
| soups: | - | 10.00000 | |
| sugar substitutes: | - | - | |
| sweet sauces: | - | - | |
Safety References:
| Flavor & Extract Manufacturers Association (FEMA) reference(s): | |
| The FEMA GRAS assessment of lactones used as flavor ingredients. View pdf | |
| European Food Safety Athority(EFSA): | Flavor usage levels; Subacute, Subchronic, Chronic and Carcinogenicity Studies; Developmental / Reproductive Toxicity Studies; Genotoxicity Studies... |
| European Food Safety Authority (EFSA) reference(s): | |
| Opinion of the Scientific Panel on Food Additives, Flavourings, Processing Aids and Materials in contact with Food (AFC) on a request from the Commission related to - Flavouring Group Evaluation 10: Aliphatic primary and secondary saturated and unsaturated alcohols, aldehydes, acetals, carboxylic acids and esters containing an additional oxygenated functional group and lactones from chemical groups 9, 13 and 30 - (Commission Regulation (EC) No 1565/2000 of 18 July 2000) View page or View pdf | |
| Flavouring Group Evaluation 10, Revision 1 (FGE10 Rev1)[1] - Aliphatic primary and secondary saturated and unsaturated alcohols, aldehydes, acetals, carboxylic acids and esters containing an additional oxygenated functional group and lactones from chemical groups 9, 13 and 30 - Scientific Opinion of the Panel on Food Additives, Flavourings, Processing Aids and Materials in Contact with Food (AFC) View page or View pdf | |
| Flavouring Group Evaluation 64 (FGE.64): Consideration of aliphatic acyclic diols, triols, and related substances evaluated by JECFA (57th meeting) structurally related to aliphatic primary and secondary saturated and unsaturated alcohols, aldehydes, acetals, carboxylic acids and esters containing an additional oxygenated functional group and lactones from chemical groups 9, 13 and 30 evaluated by EFSA in FGE.10Rev1 (EFSA, 2008ab) View page or View pdf | |
| Scientific Opinion on Flavouring Group Evaluation 92 (FGE.92): Consideration of aliphatic acyclic diols, triols, and related substances evaluated by JECFA (68th meeting) structurally related to aliphatic primary and secondary saturated and unsaturated alcohols, aldehydes, acetals, carboxylic acids and esters containing an additional oxygenated functional group and lactones evaluated by EFSA in FGE.10Rev1 (2009) View page or View pdf | |
| Scientific Opinion on Flavouring Group Evaluation 10, Revision 2 (FGE.10Rev2): Aliphatic primary and secondary saturated and unsaturated alcohols, aldehydes, acetals, carboxylic acids and esters containing an additional oxygenated functional group and lactones from chemical groups 9, 13 and 30 View page or View pdf | |
| EPI System: | View |
| NIOSH International Chemical Safety Cards: | search |
| Chemical Carcinogenesis Research Information System: | Search |
| AIDS Citations: | Search |
| Cancer Citations: | Search |
| Toxicology Citations: | Search |
| Carcinogenic Potency Database: | Search |
| EPA GENetic TOXicology: | Search |
| EPA Substance Registry Services (TSCA): | 96-48-0 |
| EPA ACToR: | Toxicology Data |
| EPA Substance Registry Services (SRS): | Registry |
| Laboratory Chemical Safety Summary : | 7302 |
| National Institute of Allergy and Infectious Diseases: | Data |
| WGK Germany: | 1 |
| oxolan-2-one | |
| Chemidplus: | 0000096480 |
| EPA/NOAA CAMEO: | hazardous materials |
| RTECS: | 96-48-0 |
References:
| oxolan-2-one | |
| NIST Chemistry WebBook: | Search Inchi |
| Canada Domestic Sub. List: | 96-48-0 |
| Pubchem (cid): | 7302 |
| Pubchem (sid): | 134972536 |
| Flavornet: | 96-48-0 |
| Pherobase: | View |
Other Information:
| (IUPAC): | Atomic Weights of the Elements 2011 (pdf) |
| Videos: | The Periodic Table of Videos |
| tgsc: | Atomic Weights use for this web site |
| (IUPAC): | Periodic Table of the Elements |
| FDA Substances Added to Food (formerly EAFUS): | View |
| CHEBI: | View |
| CHEMBL: | View |
| UM BBD: | Search |
| KEGG (GenomeNet): | C01770 |
| HMDB (The Human Metabolome Database): | HMDB00549 |
| FooDB: | FDB003392 |
| Export Tariff Code: | 2932.29.6000 |
| MedlinePlusSupp: | View |
| Typical G.C. | |
| VCF-Online: | VCF Volatile Compounds in Food |
| ChemSpider: | View |
| Wikipedia: | View |
| EFSA Update of results on the monitoring of furan levels in food: | Read Report |
| EFSA Previous report: Results on the monitoring of furan levels in food: | Read Report |
| EFSA Report of the CONTAM Panel on provisional findings on furan in food: | Read Report |
| Formulations/Preparations: •electronic-grade butyrolactone is about 99% pure . •grade: technical | |
Potential Blenders and core components note
Potential Uses:
| butterscotch | FR | |
| coconut | FR | |
| mango | FR | |
| peach | FR | |
| solvents |
Occurrence (nature, food, other): note
| beer Search PMC Picture | |
| bilberry fruit juice Search Trop Picture | |
| coffee PbMd Search PMC Picture | |
| guava fruit headspace reunion @ 7.60% Data GC Search Trop Picture | |
| lavender oil spike spain @ 0.067% Data GC Search Trop Picture | |
| mango fruit Search Trop Picture | |
| pepper bell pepper fruit Search Trop Picture | |
| pineapple fruit Search Trop Picture | |
| wine orange wine Search PMC Picture | |
| wine white wine Search PMC Picture |
Synonyms:
| agrisynth BLO | |
| 1,2- | butanolide |
| 1,4- | butanolide |
| 4- | butanolide |
| butyro-1,4-lactone | |
| 1,4- | butyrolactone |
| 4- | butyrolactone |
| g- | butyrolactone |
| butyrolactone gamma | |
| gamma- | butyrolactone natural |
| butyryl lactone | |
| 4- | deoxytetronic acid |
| dihydro-2(3H)-furanone | |
| 4,5- | dihydro-2(3H)-furanone |
| dihydrofuran-2(3H)-one | |
| furan-2(3H)-one, dihydro- | |
| 2(3H)- | furanone, dihydro- |
| 4- | hydroxy-butanoic acid g-lactone |
| 4- | hydroxybutanoic acid lactone |
| gamma- | hydroxybutyric acid cyclic ester |
| 4- | hydroxybutyric acid lactone |
| g- | hydroxybutyric acid lactone |
| gamma- | hydroxybutyric acid lactone |
| gamma- | hydroxybutyrolactone |
| oxolan-2-one | |
| 2- | oxolanone |
| tetrahydro-2-furanone | |
| 2,3,4,5- | tetrahydro-2-furanone |












