|
Category: flavoring agents
US / EU / FDA / JECFA / FEMA / FLAVIS / Scholar / Patent Information:
Physical Properties:
| Appearance: | white to yellow crystalline solid (est) |
| Assay: | 97.00 to 100.00 % sum of isomers
|
| Food Chemicals Codex Listed: | No |
| Melting Point: | 39.00 to 42.00 °C. @ 760.00 mm Hg
|
| Boiling Point: | 260.00 to 261.00 °C. @ 760.00 mm Hg
|
| Boiling Point: | 147.00 to 149.00 °C. @ 20.00 mm Hg
|
| Congealing Point: | 39.00 °C.
|
| Vapor Pressure: | 0.012000 mmHg @ 25.00 °C. (est) |
| Flash Point: | 253.00 °F. TCC ( 122.78 °C. )
|
| logP (o/w): | 2.070 |
| Soluble in: |
| | alcohol | | | water, 1345 mg/L @ 25 °C (est) |
| Stability: |
| | bath foam | | | hair spray | | | non-discoloring | | | shampoo | | | soap |
Organoleptic Properties:
| |
| Odor Type: spicy |
| |
| Odor Strength: | medium ,
recommend smelling in a 1.00 % solution or less |
| |
| Substantivity: | 400 hour(s) at 20.00 % |
| |
| | sweet spicy cinnamon balsamic rhubarb creamy floral |
Odor Description:
at 1.00 % in dipropylene glycol. | sweet spice cinnamon balsam rhubarb creamy floral
Luebke, William tgsc, (1986) |
| |
| | sweet spicy cinnamon balsamic anisic powdery phenolic jammy fruity |
Odor Description:
| Sweet, spicy, cinnamon, balsamic, anisyl, powdery and phenolic with jammy, fruity notes
Mosciano, Gerard P&F 22, No. 3, 47, (1997) |
| |
| |
| Flavor Type: spicy |
| |
| | spicy cinnamyl powdery waxy balsamic |
Taste Description:
at 30.00 ppm. | Spicy, cinnamate-like with powdery and waxy balsamic nuances
Mosciano, Gerard P&F 22, No. 3, 47, (1997) |
| |
| Odor and/or flavor descriptions from others (if found). |
| |
| Sigma-Aldrich |
| Benzylideneacetone, ≥98%, FG |
| Odor Description: | almond; anise; balsam; butter; cherry; cinnamon; creamy; jam; floral; herbaceous; sweet; vanilla; woody |
| |
| |
Cosmetic Information:
Suppliers:
Safety Information:
| Preferred SDS: View |
| European information : |
| Most important hazard(s): | | Xi - Irritant |
R 36/37/38 - Irritating to eyes, respiratory system, and skin.
R 43 - May cause sensitisation by skin contact.
S 02 - Keep out of the reach of children.
S 22 - Do not breath dust.
S 26 - In case of contact with eyes, rinse immediately with plenty of water and seek medical advice.
S 37/39 - Wear suitable gloves and eye/face protection.
|
| |
| Hazards identification |
| |
| Classification of the substance or mixture |
| GHS Classification in accordance with 29 CFR 1910 (OSHA HCS) |
| None found. |
| GHS Label elements, including precautionary statements |
| |
| Pictogram | |
| |
| Hazard statement(s) |
| None found. |
| Precautionary statement(s) |
| None found. |
| Human Experience: |
| 2 % solution: no irritation or sensitization. |
| Oral/Parenteral Toxicity: |
oral-rat LD50 2031 mg/kg
VASCULAR: REGIONAL OR GENERAL ARTERIOLAR OR VENOUS DILATION
BEHAVIORAL: MUSCLE WEAKNESS
SKIN AND APPENDAGES (SKIN): HAIR: OTHER
United States Environmental Protection Agency, Office of Pesticides and Toxic Substances. Vol. 8EHQ-0391-1194
intraperitoneal-mouse LD50 1210 mg/kg
Arzneimittel-Forschung. Drug Research. Vol. 19, Pg. 617, 1969.
intravenous-mouse LD50 112 mg/kg
Arzneimittel-Forschung. Drug Research. Vol. 19, Pg. 617, 1969.
|
| Dermal Toxicity: |
|
Not determined
|
| Inhalation Toxicity: |
|
Not determined
|
Safety in Use Information:
| Category: | flavoring agents |
| IFRA Critical Effect: | Dermal sensitization |
| IFRA: | View Standard |
| Recommendation for benzylidene acetone usage levels up to: | | | PROHIBITED: Should not be used as a fragrance ingredient.
|
| |
| Maximised Survey-derived Daily Intakes (MSDI-EU): | 1.20 (μg/capita/day) |
| Maximised Survey-derived Daily Intakes (MSDI-USA): | 7.00 (μg/capita/day) |
| Modified Theoretical Added Maximum Daily Intake (mTAMDI): | 1100 (μg/person/day) |
| Threshold of Concern: | 1800 (μg/person/day) |
| Structure Class: | I |
| Use levels for FEMA GRAS flavoring substances on which the FEMA Expert Panel based its judgments that the substances are generally recognized as safe (GRAS). |
| The Expert Panel also publishes separate extensive reviews of scientific information on all FEMA GRAS flavoring substances and can be found at FEMA Flavor Ingredient Library |
| publication number: 3 |
| Click here to view publication 3 |
| | average usual ppm | average maximum ppm |
| baked goods: | - | 4.50000 |
| beverages(nonalcoholic): | - | 0.82000 |
| beverages(alcoholic): | - | - |
| breakfast cereal: | - | - |
| cheese: | - | - |
| chewing gum: | - | - |
| condiments / relishes: | - | - |
| confectionery froastings: | - | - |
| egg products: | - | - |
| fats / oils: | - | 0.20000 |
| fish products: | - | - |
| frozen dairy: | - | 0.84000 |
| fruit ices: | - | 0.84000 |
| gelatins / puddings: | - | 2.10000 |
| granulated sugar: | - | - |
| gravies: | - | - |
| hard candy: | - | 3.70000 |
| imitation dairy: | - | - |
| instant coffee / tea: | - | - |
| jams / jellies: | - | - |
| meat products: | - | - |
| milk products: | - | - |
| nut products: | - | - |
| other grains: | - | - |
| poultry: | - | - |
| processed fruits: | - | - |
| processed vegetables: | - | - |
| reconstituted vegetables: | - | - |
| seasonings / flavors: | - | - |
| snack foods: | - | - |
| soft candy: | - | - |
| soups: | - | - |
| sugar substitutes: | - | - |
| sweet sauces: | - | - |
| |
| Food categories according to Commission Regulation EC No. 1565/2000 (EC, 2000) in FGE.06 (EFSA, 2002a). According to the Industry the "normal" use is defined as the average of reported usages and "maximum use" is defined as the 95th percentile of reported usages (EFSA, 2002i). |
| Note: mg/kg = 0.001/1000 = 0.000001 = 1/1000000 = ppm. |
| | average usage mg/kg | maximum usage mg/kg |
| Dairy products, excluding products of category 02.0 (01.0): | 1.59000 | 2.90000 |
| Fats and oils, and fat emulsions (type water-in-oil) (02.0): | 0.02000 | 0.20000 |
| Edible ices, including sherbet and sorbet (03.0): | - | - |
| Processed fruit (04.1): | - | - |
| Processed vegetables (incl. mushrooms & fungi, roots & tubers, pulses and legumes), and nuts & seeds (04.2): | - | - |
| Confectionery (05.0): | 4.44000 | 8.72000 |
| Chewing gum (05.0): | 1.59000 | 2.90000 |
| Cereals and cereal products, incl. flours & starches from roots & tubers, pulses & legumes, excluding bakery (06.0): | 5.25000 | 10.00000 |
| Bakery wares (07.0): | - | - |
| Meat and meat products, including poultry and game (08.0): | - | - |
| Fish and fish products, including molluscs, crustaceans and echinoderms (MCE) (09.0): | - | - |
| Eggs and egg products (10.0): | - | - |
| Sweeteners, including honey (11.0): | - | - |
| Salts, spices, soups, sauces, salads, protein products, etc. (12.0): | - | - |
| Foodstuffs intended for particular nutritional uses (13.0): | - | - |
| Non-alcoholic ("soft") beverages, excl. dairy products (14.1): | 0.89000 | 1.72000 |
| Alcoholic beverages, incl. alcohol-free and low-alcoholic counterparts (14.2): | 1.28000 | 1.92000 |
| Ready-to-eat savouries (15.0): | - | - |
| Composite foods (e.g. casseroles, meat pies, mincemeat) - foods that could not be placed in categories 01.0 - 15.0 (16.0): | - | - |
Safety References:
| Flavor & Extract Manufacturers Association (FEMA) reference(s): |
| The FEMA GRAS assessment of aromatic substituted secondary alcohols, ketones, and related esters used as flavor ingredients. View pdf |
| European Food Safety Athority(EFSA): | Flavor usage levels; Subacute, Subchronic, Chronic and Carcinogenicity Studies; Developmental / Reproductive Toxicity Studies; Genotoxicity Studies... |
| European Food Safety Authority (EFSA) reference(s): |
List of apha, beta-Unsaturated Aldehydes and Ketones representative of FGE.19 substances for Genotoxicity Testing [1] - Statement of the Panel on Food Contact Materials, Enzymes, Flavourings and Processing Aids (CEF)
View page or View pdf |
Scientific Opinion on Flavouring Group Evaluation 215 (FGE.215): Seven a,ß-Unsaturated Cinnamyl Ketones from subgroup 3.2 of FGE.19
View page or View pdf |
Scientific Opinion on Flavouring Group Evaluation 215 Revision 1 (FGE.215Rev1): seven a,ß-unsaturated cinnamyl ketones from subgroup 3.2 of FGE.19
View page or View pdf |
Safety and efficacy of 4-phenylbut-3-en-2-one and benzophenone belonging to chemical group 21 when used as flavouring compounds for all animal species
View page or View pdf |
Scientific Opinion on Flavouring Group Evaluation 69, Revision 1 (FGE.69Rev1): consideration of aromatic substituted secondary alcohols, ketones and related esters evaluated by JECFA (57th meeting), structurally related to aromatic ketones from chemical group 21 evaluated by EFSA in FGE.16Rev2
View page or View pdf |
| EPI System: | View |
| Chemical Carcinogenesis Research Information System: | Search |
| AIDS Citations: | Search |
| Cancer Citations: | Search |
| Toxicology Citations: | Search |
| EPA Substance Registry Services (TSCA): | 122-57-6 |
| EPA ACToR: | Toxicology Data |
| EPA Substance Registry Services (SRS): | Registry |
| Laboratory Chemical Safety Summary : | 15909 |
| National Institute of Allergy and Infectious Diseases: | Data |
| WGK Germany: | 1 |
| | 4-phenylbut-3-en-2-one |
| Chemidplus: | 0000122576 |
| RTECS: | EN0330050 for cas# 122-57-6 |
References:
Other Information:
Potential Blenders and core components note
| |
| For Odor |
| No odor group found for these |
| | dehydro-ar-ionene | |
| alpha- | ionone | FL/FR |
| acidic |
| | cyclohexyl acetic acid | FL/FR |
| amber |
| | ambrette seed oil | FL/FR |
| anise |
| | anise oleoresin | FL/FR |
| | anise seed oil | FL/FR |
| star | anise seed oil china | FL/FR |
| | anise seed oil colombia | FL/FR |
| star | anise seed oil terpeneless | FL/FR |
| sweet | fennel absolute | FL/FR |
| sweet | fennel oleoresin | FL/FR |
| anisic |
| ortho- | acetanisole | FL/FR |
| para- | acetanisole | FL/FR |
| | amyl furoate | FL/FR |
| para- | anisaldehyde | FL/FR |
| ortho- | anisaldehyde | FL/FR |
| para- | anisyl phenyl acetate | FL/FR |
| para- | anisyl propanal | FR |
| | dihydroanethol | FL/FR |
| | ethyl para-anisate | FL/FR |
| | ocimum basilicum herb oil | FL/FR |
| | sassafras acetate | FL/FR |
| balsamic |
| sumatra | benzoin absolute | FL/FR |
| sumatra | benzoin resinoid | FL/FR |
| | benzophenone | FR |
| alpha- | bisabolene | FL/FR |
| iso | butyl benzoate | FL/FR |
| iso | butyl cinnamate | FL/FR |
| | cinnamyl alcohol | FL/FR |
| | cinnamyl benzoate | FL/FR |
| | cinnamyl butyrate | FL/FR |
| | cinnamyl cinnamate | FL/FR |
| | copaiba balsam oil | FL/FR |
| | ethyl cinnamate | FL/FR |
| | frankincense absolute | FL/FR |
| (Z)-3- | hexen-1-yl cinnamate | FR |
| | linalyl cinnamate | FL/FR |
| | myrrh resinoid | FR |
| | octyl cinnamate | FR |
| | opoponax oil (balsamodendron kafal) | FL/FR |
| | opoponax resinoid (balsamodendron kafal) | FR |
| | peru balsam oil | FL/FR |
| | peru balsam resinoid | FL/FR |
| 2- | phenoxyethyl formate | FR |
| 3- | phenyl propyl acetate | FL/FR |
| black | poplar bud oleoresin | FL/FR |
| | tetrahydrofurfuryl cinnamate | FL/FR |
| | tolu balsam oil | FL/FR |
| chocolate |
| | cocoa oleoresin | FL/FR |
| | cocoa pentenal | FL/FR |
| citrus |
| | citral | FL/FR |
| | citronella oil ceylon | FL/FR |
| | lemongrass oil | FL/FR |
| | litsea cubeba fruit oil | FL/FR |
| creamy |
| para- | vanillic acid | FL/FR |
| floral |
| | anise indene | FR |
| para- | anisyl acetaldehyde | FL/FR |
| | anisyl propanal / methyl anthranilate schiff's base | FR |
| | autumn carboxylate | FR |
| | citronellal | FL/FR |
| | dimethyl alpha-ionone | FR |
| | dimethyl anthranilate | FL/FR |
| | geranyl phenyl acetate | FL/FR |
| | hawthorn acetate | FR |
| | heliotropin | FL/FR |
| | hydroxycitronellal | FL/FR |
| alpha- | ionone | FL/FR |
| (E)-beta- | ionone | FL/FR |
| alpha- | irone | FL/FR |
| ortho- | methyl acetophenone | FL/FR |
| beta-iso | methyl ionone | FL/FR |
| alpha-iso | methyl ionone (50% min.) | FL/FR |
| alpha-iso | methyl ionone (90% min.) | FL/FR |
| | methyl ionyl acetate | FL/FR |
| | orris rhizome resinoid (iris pallida) | FL/FR |
| iso | propyl phenyl acetate | FL/FR |
| | rhodinol | FL/FR |
| | rhodinyl phenyl acetate | FL/FR |
| (E)-2,5,9- | trimethyl-4,9-decadien-1-al | FR |
| fruity |
| | acetyl methyl anthranilate | FL/FR |
| para- | anisyl methyl ketone | FL/FR |
| | berry hexanoate | FR |
| | cherry oxyacetate | FL/FR |
| | ethyl methyl-para-tolyl glycidate | FL/FR |
| (E,E)- | ethyl sorbate | FL/FR |
| | peach pivalate | FR |
| | tolualdehydes (mixed o,m,p) | FL/FR |
| green |
| | diphenyl oxide | FL/FR |
| para- | methyl hydratropaldehyde | FL/FR |
| | phenoxyethyl isobutyrate | FL/FR |
| | tiglaldehyde | FL/FR |
| herbal |
| american | elder flower absolute | FR |
| | floral nitrile | FR |
| licorice |
| (E)- | anethol | FL/FR |
| | clausena anisata leaf oil | |
| minty |
| | ethyl salicylate | FL/FR |
| bitter | fennel flower oil lithuania | |
| bitter | fennel seed oil lithuania | CS |
| musk |
| | cyclohexadecanone | FR |
| | ethylene brassylate | FL/FR |
| | exaltone (Firmenich) | FR |
| dextro,laevo- | muscone | FL/FR |
| | musk amberol | FR |
| | musk indane | FR |
| | musk tetralin | FL/FR |
| omega- | pentadecalactone | FL/FR |
| musty |
| | cocoa butenal | FL/FR |
| naphthyl |
| para- | methyl anisole | FL/FR |
| beta- | naphthyl ethyl ether | FL/FR |
| nutty |
| 2,3,5- | trimethyl pyrazine | FL/FR |
| pine |
| white | pine bark oil | FL/FR |
| powdery |
| para- | anisyl acetate | FL/FR |
| para- | anisyl alcohol | FL/FR |
| alpha- | methyl ionone | FL/FR |
| spicy |
| | artemisia dracunculus herb oil | FL/FR |
| | bay leaf oil terpeneless | FL/FR |
| | benzyl isoeugenol | FL/FR |
| | cassia bark oil china | FL/FR |
| | cinnamyl acetate | FL/FR |
| | cinnamyl propionate | FL/FR |
| | clove bud oil | FL/FR |
| | mace oil CO2 extract | FL/FR |
| para- | methoxy-alpha-methyl cinnamaldehyde | FL/FR |
| para- | methoxycinnamaldehyde | FL/FR |
| alpha- | methyl cinnamaldehyde | FL/FR |
| | methyl heptadienone | FL/FR |
| alpha- | methyl-(E)-cinnamaldehyde | FL/FR |
| | myrtenal | FL/FR |
| | nutmeg absolute | FL/FR |
| | nutmeg oil CO2 extract | FL/FR |
| | spicy acetoacetate | FL/FR |
| terpenic |
| alpha- | terpineol | FL/FR |
| | mint lactone | FL/FR |
| | tonka undecanone | FR |
| vanilla |
| | heliotropyl alcohol | FL/FR |
| | vanillyl acetate | FL/FR |
| | vanillylidene acetone | FL/FR |
| woody |
| | amber formate | FR |
| | guaiacwood oil | FL/FR |
| | patchouli ethanone | FR |
| (+)-alpha- | santalyl acetate | FL/FR |
| | timber propanol | FR |
| | vetiveryl acetate | FL/FR |
| | woody epoxide | FR |
| | woody octene | FR |
| |
| For Flavor |
| |
| No flavor group found for these |
| | amyl furoate | FL/FR |
| para- | anisyl acetaldehyde | FL/FR |
| alpha- | bisabolene | FL/FR |
| | cinnamyl benzoate | FL/FR |
| | clausena anisata leaf oil | |
| | dehydro-ar-ionene | |
| 4- | ethyl anisole | FL |
| bitter | fennel flower oil lithuania | |
| 2- | furfurylidene butyraldehyde | FL |
| | heliotropyl alcohol | FL/FR |
| alpha- | ionone | FL/FR |
| para- | methoxy-alpha-methyl cinnamaldehyde | FL/FR |
| | methyl furfuracrylate | FL |
| beta-iso | methyl ionone | FL/FR |
| white | pine bark oil | FL/FR |
| black | poplar bud oleoresin | FL/FR |
| (+)-alpha- | santalyl acetate | FL/FR |
| | tetrahydrofurfuryl cinnamate | FL/FR |
| | vetiveryl acetate | FL/FR |
| amber |
| | ambrette seed oil | FL/FR |
| | musk tetralin | FL/FR |
| anise |
| (E)- | anethol | FL/FR |
| | anise oleoresin | FL/FR |
| | anise seed oil | FL/FR |
| star | anise seed oil china | FL/FR |
| | anise seed oil colombia | FL/FR |
| star | anise seed oil terpeneless | FL/FR |
| | ethyl para-anisate | FL/FR |
| sweet | fennel absolute | FL/FR |
| sweet | fennel oleoresin | FL/FR |
| anisic |
| para- | acetanisole | FL/FR |
| ortho- | anisaldehyde | FL/FR |
| para- | anisyl phenyl acetate | FL/FR |
| ortho- | methyl acetophenone | FL/FR |
| balsamic |
| sumatra | benzoin resinoid | FL/FR |
| iso | butyl cinnamate | FL/FR |
| | copaiba balsam oil | FL/FR |
| | ethyl cinnamate | FL/FR |
| | opoponax oil (balsamodendron kafal) | FL/FR |
| | peru balsam oil | FL/FR |
| | peru balsam resinoid | FL/FR |
| 3- | phenyl propyl acetate | FL/FR |
| | tolu balsam oil | FL/FR |
| | vanillylidene acetone | FL/FR |
| cherry |
| | heliotropin | FL/FR |
| para- | methoxycinnamaldehyde | FL/FR |
| chocolate |
| | cocoa oleoresin | FL/FR |
| citrus |
| | citral | FL/FR |
| | citronella oil ceylon | FL/FR |
| | lemongrass oil | FL/FR |
| | litsea cubeba fruit oil | FL/FR |
| alpha- | terpineol | FL/FR |
| coconut |
| 6- | methyl coumarin | FL |
| creamy |
| para- | anisaldehyde | FL/FR |
| | dihydrocoumarin | FL |
| | mint lactone | FL/FR |
| para- | vanillic acid | FL/FR |
| floral |
| | cinnamyl propionate | FL/FR |
| | citronellal | FL/FR |
| | cocoa pentenal | FL/FR |
| | geranyl phenyl acetate | FL/FR |
| alpha- | ionone | FL/FR |
| alpha-iso | methyl ionone (50% min.) | FL/FR |
| alpha-iso | methyl ionone (90% min.) | FL/FR |
| | orris rhizome resinoid (iris pallida) | FL/FR |
| | rhodinol | FL/FR |
| fruity |
| | acetyl methyl anthranilate | FL/FR |
| para- | anisyl acetate | FL/FR |
| para- | anisyl alcohol | FL/FR |
| iso | butyl benzoate | FL/FR |
| | cherry oxyacetate | FL/FR |
| | dimethyl anthranilate | FL/FR |
| | ethyl methyl-para-tolyl glycidate | FL/FR |
| (E,E)- | ethyl sorbate | FL/FR |
| | linalyl cinnamate | FL/FR |
| alpha- | methyl ionone | FL/FR |
| | tiglaldehyde | FL/FR |
| | tolualdehydes (mixed o,m,p) | FL/FR |
| green |
| | cinnamyl alcohol | FL/FR |
| | cocoa butenal | FL/FR |
| | diphenyl oxide | FL/FR |
| | methyl heptadienone | FL/FR |
| para- | methyl hydratropaldehyde | FL/FR |
| | phenoxyethyl isobutyrate | FL/FR |
| herbal |
| | dihydroanethol | FL/FR |
| | ocimum basilicum herb oil | FL/FR |
| honey |
| iso | propyl phenyl acetate | FL/FR |
| licorice |
| 2- | butyl-3-methyl pyrazine | FL |
| meaty |
| ortho- | thioguaiacol | FL |
| medicinal |
| | frankincense absolute | FL/FR |
| minty |
| | ethyl salicylate | FL/FR |
| | myrtenal | FL/FR |
| (1R)-(-)- | myrtenal | FL |
| musk |
| | ethylene brassylate | FL/FR |
| dextro,laevo- | muscone | FL/FR |
| musty |
| 2,3,5- | trimethyl pyrazine | FL/FR |
| naphthyl |
| para- | methyl anisole | FL/FR |
| nutty |
| | furfural acetone | FL |
| powdery |
| ortho- | acetanisole | FL/FR |
| beta- | naphthyl ethyl ether | FL/FR |
| | powdery ketone | FL |
| spicy |
| para- | anisyl methyl ketone | FL/FR |
| | artemisia dracunculus herb oil | FL/FR |
| | bay leaf oil terpeneless | FL/FR |
| sumatra | benzoin absolute | FL/FR |
| | benzyl isoeugenol | FL/FR |
| | cassia bark oil china | FL/FR |
| | cinnamyl acetate | FL/FR |
| | cinnamyl cinnamate | FL/FR |
| | clove bud oil | FL/FR |
| | galangal root oleoresin | FL |
| | mace oil CO2 extract | FL/FR |
| alpha- | methyl cinnamaldehyde | FL/FR |
| alpha- | methyl-(E)-cinnamaldehyde | FL/FR |
| | nutmeg absolute | FL/FR |
| | nutmeg oil CO2 extract | FL/FR |
| | spicy acetoacetate | FL/FR |
| sweet |
| | cyclohexyl acetic acid | FL/FR |
| tarragon |
| | sassafras acetate | FL/FR |
| vanilla |
| omega- | pentadecalactone | FL/FR |
| | vanillyl acetate | FL/FR |
| waxy |
| | hydroxycitronellal | FL/FR |
| | rhodinyl phenyl acetate | FL/FR |
| winey |
| | cinnamyl butyrate | FL/FR |
| woody |
| | guaiacwood oil | FL/FR |
| (E)-beta- | ionone | FL/FR |
| alpha- | irone | FL/FR |
| | methyl ionyl acetate | FL/FR |
| |
Potential Uses:
Occurrence (nature, food, other): noteSynonyms:
| | acetocinnamone | | | benzal acetone | | | benzalacetone | | | benzylideneacetone | | 3- | buten-2-one, 4-phenyl- | | | methyl 2-phenyl vinyl ketone | | | methyl beta-styryl ketone | | | methyl styryl ketone | | 4- | phenyl but-3-en-2-one | | 4- | phenyl butenone | | 2- | phenyl vinyl methyl ketone | | 4- | phenyl-3-buten-2-one | | 4- | phenylbut-3-en-2-one | | 4- | phenylbutenone | | | styryl methyl ketone |
Articles:
| PubMed: | Digallane with redox-active diimine ligand: dualism of electron-transfer reactions. |
| PubMed: | Ferrous Carbonyl Dithiolates as Precursors to FeFe, FeCo, and FeMn Carbonyl Dithiolates. |
| PubMed: | Additive-assisted regioselective 1,3-dipolar cycloaddition of azomethine ylides with benzylideneacetone. |
| PubMed: | Protein preparation, crystallization and preliminary X-ray analysis of Polygonum cuspidatum bifunctional chalcone synthase/benzalacetone synthase. |
| PubMed: | An entomopathogenic bacterium, Xenorhabdus nematophila, suppresses expression of antimicrobial peptides controlled by Toll and Imd pathways by blocking eicosanoid biosynthesis. |
| PubMed: | (99m)Tc-labeled dibenzylideneacetone derivatives as potential SPECT probes for in vivo imaging of β-amyloid plaque. |
| PubMed: | Metabolism of BYZX in human liver microsomes and cytosol: identification of the metabolites and metabolic pathways of BYZX. |
| PubMed: | Engineering of plant type III polyketide synthases. |
| PubMed: | Gas phase retro-Michael reaction resulting from dissociative protonation: fragmentation of protonated warfarin in mass spectrometry. |
| PubMed: | Gas chromatography with flame ionization detection for determination of additives in an electrolytic Zn bath. |
| PubMed: | Toxicology and carcinogenesis studies of methyl trans-styryl ketone (CAS NO 1896-62-4) in F344/N rats and B6C3F1 mice (feed and dermal studies). |
| PubMed: | Phospholipase A2 inhibitors synthesized by two entomopathogenic bacteria, Xenorhabdus nematophila and Photorhabdus temperata subsp. temperata. |
| PubMed: | A facile solvent free Claisen-Schmidt reaction: synthesis of α,α'-bis-(substituted-benzylidene)cycloalkanones and α,α'-bis-(substituted-alkylidene)cycloalkanones. |
| PubMed: | On the accuracy of DFT methods in reproducing ligand substitution energies for transition metal complexes in solution: the role of dispersive interactions. |
| PubMed: | Synthesis of the pyridinyl analogues of dibenzylideneacetone (pyr-dba) via an improved Claisen-Schmidt condensation, displaying diverse biological activities as curcumin analogues. |
| PubMed: | Evaluation of hepatic glutathione transferase Mu 1 and Theta 1 activities in humans and mice using genotype information. |
| PubMed: | Ni(II) and Pd(II) pyridinyloxazolidine-compounds: synthesis, X-ray characterisation and catalytic activities in the aza-Michael reaction. |
| PubMed: | Bacterial metabolites of an entomopathogenic bacterium, Xenorhabdus nematophila, inhibit a catalytic activity of phenoloxidase of the diamondback moth, Plutella xylostella. |
| PubMed: | A New Route to Azadithiolato Complexes. |
| PubMed: | Pd-NHC catalyzed conjugate addition versus the Mizoroki-Heck reaction. |
| PubMed: | Benzylideneacetone, an eicosanoid biosynthesis inhibitor enhances baculovirus pathogenicity in the diamondback moth, Plutella xylostella. |
| PubMed: | A structure-based mechanism for benzalacetone synthase from Rheum palmatum. |
| PubMed: | Synthesis and anti-inflammatory activity of novel (substituted)benzylidene acetone oxime ether derivatives: molecular modeling study. |
| PubMed: | Quantitative structure-activity relationship modeling of antioxidant activities of hydroxybenzalacetones using quantum chemical, physicochemical and spatial descriptors. |
| PubMed: | Highly enantioselective organocatalytic conjugate addition of nitromethane to benzylidene acetones. |
| PubMed: | Crystallization and preliminary crystallographic analysis of a plant type III polyketide synthase that produces benzalacetone. |
| PubMed: | Benzylideneacetone, an immunosuppressant, enhances virulence of Bacillus thuringiensis against beet armyworm (Lepidoptera: Noctuidae). |
| PubMed: | New "green" approaches to the synthesis of pyrazole derivatives. |
| PubMed: | Microbial production of 4-hydroxybenzylidene acetone, the direct precursor of raspberry ketone. |
| PubMed: | Novel hydrido-ruthenium(II) complexes with histidine derivatives and their application in the hydrogenation of ketones. |
| PubMed: | Phenolic Michael reaction acceptors: combined direct and indirect antioxidant defenses against electrophiles and oxidants. |
| PubMed: | An entomopathogenic bacterium, Xenorhabdus nematophila, inhibits hemocyte phagocytosis of Spodoptera exigua by inhibiting phospholipase A(2). |
| PubMed: | Structure function analysis of benzalacetone synthase from Rheum palmatum. |
| PubMed: | Highly asymmetric Michael addition to alpha,beta-unsaturated ketones catalyzed by 9-amino-9-deoxyepiquinine. |
| PubMed: | Concise asymmetric total synthesis of obolactone. |
| PubMed: | Antioxidant effects of hydroxybenzalacetones on peroxynitrite-induced lipid peroxidation in red blood cell membrane ghost and SOS response in Salmonella typhimurium TA4107/pSK1002. |
| PubMed: | Metabolism of the alpha,beta-unsaturated ketones, chalcone and trans-4-phenyl-3-buten-2-one, by rat liver microsomes and estrogenic activity of the metabolites. |
| PubMed: | Reaction of metalated nitriles with enones. |
| PubMed: | Solution and solid state structure and tautomerism of azo coupled enaminone derivatives of benzoylacetone. |
| PubMed: | Highly enantioselective conjugate additions of potassium organotrifluoroborates to enones by use of monodentate phosphoramidite ligands. |
| PubMed: | Quantitative structure-activity relationship analyses of antioxidant and free radical scavenging activities for hydroxybenzalacetones. |
| PubMed: | Identification of an antibacterial compound, benzylideneacetone, from Xenorhabdus nematophila against major plant-pathogenic bacteria. |
| PubMed: | Site-directed mutagenesis of benzalacetone synthase. The role of the Phe215 in plant type III polyketide synthases. |
| PubMed: | Organocatalytic asymmetric conjugate addition of nitroalkanes to alpha,beta-unsaturated enones using novel imidazoline catalysts. |
| PubMed: | Enzymatic formation of an unnatural C(6)-C(5) aromatic polyketide by plant type III polyketide synthases. |
| PubMed: | Quantum chemical- and 3-D-QSAR (CoMFA) studies of benzalacetones and 1,1,1-trifluoro-4-phenyl-3-buten-2-ones. |
| PubMed: | Reductive metabolism of an alpha,beta-ketoalkyne, 4-phenyl-3-butyn-2-one, by rat liver preparations. |
| PubMed: | Role of 4-phenyl-3-buten-2-one in boar taint: identification of new compounds related to sensorial descriptors in pig fat. |
| PubMed: | Potency of Michael reaction acceptors as inducers of enzymes that protect against carcinogenesis depends on their reactivity with sulfhydryl groups. |
| PubMed: | Structure-antimutagenic activity relationships of benzalacetone derivatives against UV-induced mutagenesis in E. coli WP2uvrA and gamma-induced mutagenesis in Salmonella typhimurium TA2638. |
| PubMed: | Production and elicitation of benzalacetone and the raspberry ketone in cell suspension cultures of Rubus idaeus. |
| PubMed: | Structure-activity relationship in potentially anti-tumor promoting benzalacetone derivatives, as assayed by the epstein-barr virus early antigen activation. |
| PubMed: | Microsomal carbonyl reductase responsible for reduction of 4-phenyl-3-buten-2-one in rats. |
| PubMed: | Reductive metabolism In vivo of trans-4-phenyl-3-buten-2-one in rats and dogs. |
| PubMed: | Anticandidial effect of phenylbutene derivatives and their interaction with ergosterol. |
| PubMed: | The three-dimensional structure of an avian class-mu glutathione S-transferase, cGSTM1-1 at 1.94 A resolution. |
| PubMed: | The inhibition by flavonoids of 2-amino-3-methylimidazo[4,5-f]quinoline metabolic activation to a mutagen: a structure-activity relationship study. |
| PubMed: | Absorption, disposition, and metabolism of trans-methyl styryl ketone in female B6C3F1 mice. |
| PubMed: | Antimutagenic effects of dehydrozingerone and its analogs on UV-induced mutagenesis in Escherichia coli. |
| PubMed: | Oral and topical absorption, disposition kinetics, and the metabolic fate of trans-methyl styryl ketone in the male Fischer 344 rat. |
| PubMed: | Glutathione transferase mimics: micellar catalysis of an enzymic reaction. |
| PubMed: | Distribution of glutathione S-transferase isoforms in rat liver after induction by beta-naphthoflavone or 3-methylcholanthrene. |
| PubMed: | Multiplicity of rat liver 15-ketoprostaglandin delta 13-reductases. |
| PubMed: | Glutathione S-transferases in the Japanese quail: tissue distribution and purification of the liver isozymes. |
| PubMed: | Stereoselective catalysis of a retro-Michael reaction by class mu glutathione transferases. Consequences for the internal distribution of products in the active site. |
| PubMed: | The high non-enzymatic conjugation rates of some glutathione S-transferase (GST) substrates at high glutathione concentrations. |
| PubMed: | Biochemical analysis of recombinant glutathione S-transferase of Fasciola hepatica. |
| PubMed: | Rational reconstruction of the active site of a class mu glutathione S-transferase. |
| PubMed: | Influences of dietary deoxycholic acid on progression of hepatocellular neoplasms and expression of glutathione S-transferases in rats. |
| PubMed: | Reduced expression of glutathione S-transferase Yb2 during progression of chemically induced hepatocellular carcinomas in Fischer 344 rats. |
| PubMed: | Effects of age and dietary restriction on liver glutathione transferase activities in Lobund-Wistar rats. |
| PubMed: | Purification and kinetic mechanism of the glutathione S-transferase from C6/36, an Aedes albopictus cell line. |
| PubMed: | Inter-individual variability of human hepatic glutathione S-transferase isozymes assessed by inhibitory capacity. |
| PubMed: | Structure and function of the xenobiotic substrate binding site of a glutathione S-transferase as revealed by X-ray crystallographic analysis of product complexes with the diastereomers of 9-(S-glutathionyl)-10-hydroxy-9,10-dihydrophenanthrene. |
| PubMed: | Human Mu-class glutathione S-transferases present in liver, skeletal muscle and testicular tissue. |
| PubMed: | Purification and characterization of class mu glutathione S-transferase isozymes from rabbit hepatic tissue. |
| PubMed: | Characterization of a novel microsomal glutathione S-transferase produced by Aspergillus ochraceus TS. |
| PubMed: | Schistosoma mansoni: single-step purification and characterization of glutathione S-transferase isoenzyme 4. |
| PubMed: | Contribution of tyrosine 6 to the catalytic mechanism of isoenzyme 3-3 of glutathione S-transferase. |
| PubMed: | Are the histidine residues of glutathione S-transferase important in catalysis? An assessment by 13C NMR spectroscopy and site-specific mutagenesis. |
| PubMed: | A novel glutathione transferase (13-13) isolated from the matrix of rat liver mitochondria having structural similarity to class theta enzymes. |
| PubMed: | Hepatic glutathione S-transferases in mice fed on a diet containing the anticarcinogenic antioxidant butylated hydroxyanisole. Isolation of mouse glutathione S-transferase heterodimers by gradient elution of the glutathione-Sepharose affinity matrix. |
| PubMed: | Variation in the expression of Mu-class glutathione S-transferase isoenzymes from human skeletal muscle. Evidence for the existence of heterodimers. |
| PubMed: | The isolation and characterization of the major glutathione S-transferase from the squid Loligo vulgaris. |
| PubMed: | Xenobiotic-inducible expression of murine glutathione S-transferase Ya subunit gene is controlled by an electrophile-responsive element. |
| PubMed: | S-(1,2-dicarboxyethyl)glutathione and activity for its synthesis in rat tissues. |
| PubMed: | Effect of aflatoxin B1 treatment in vivo on the in vitro activity of hepatic and extrahepatic glutathione S-transferase. |
| PubMed: | Sex differences in the subunits of glutathione-S-transferase isoenzyme from rat and human kidney. |
| PubMed: | Changes in hepatic cytosolic glutathione S-transferase enzymes induced by clotrimazole treatment in rats. |
| PubMed: | Purification and properties of glutathione transferase from Issatchenkia orientalis. |
| PubMed: | Differences in the influence of diet on hepatic glutathione S-transferase activity and glutathione content between young and old C57 black female mice. |
| PubMed: | Xenobiotic metabolizing enzyme systems in test fish. III. Comparative studies of liver cytosolic glutathione S-transferases. |
| PubMed: | Fluorometric determination of benzylideneacetone in fragrance products by liquid chromatography with post-column derivatization. |
| PubMed: | Purification of three cytosolic glutathione S-transferases from adult Schistosoma mansoni. |
| PubMed: | Halothane: inhibition and activation of rat hepatic glutathione S-transferases. |
| PubMed: | Paradoxical inhibition of rat glutathione transferase 4-4 by indomethacin explained by substrate-inhibitor-enzyme complexes in a random-order sequential mechanism. |
| PubMed: | Differential induction of rat hepatic glutathione S-transferase isoenzymes by hexachlorobenzene and benzyl isothiocyanate. Comparison with induction by phenobarbital and 3-methylcholanthrene. |
| PubMed: | Sex- and substrate-dependent changes in hepatic cytosolic glutathione S-transferase enzymes produced by dietary choline-deficiency. |
| PubMed: | The purification and characterization of glutathione S-transferase from the hepatopancreas of the blue crab, Callinectes sapidus. |
| PubMed: | Synthesis and antiarrhythmic activity of new 3-[2-(omega-aminoalkoxy)phenoxy]-4-phenyl-3-buten-2-ones and related compounds. |
| PubMed: | The major isozyme of rat cardiac glutathione transferases. Its correspondence to hepatic transferase X. |
| PubMed: | Kinetic independence of the subunits of cytosolic glutathione transferase from the rat. |
| PubMed: | Synthesis of 3-(4,4-dimethyl-2-piperidon-6-yl)methyl-2-pyrazoline derivatives. |
| PubMed: | Effects of pH on weak and positive control mutagens in the Ames Salmonella plate assay. |
| PubMed: | Antigenic competition in the induction of contact sensitivity in the guinea pig. |
| PubMed: | Age-development and inducibility of hepatic glutathione S-transferase activities in mice, rats, rabbits and guinea-pigs. |
| PubMed: | Purification and characterization of glutathione S-transferases P, S and N. Isolation from rat liver of Yb1 Yn protein, the existence of which was predicted by subunit hybridization in vitro. |
| PubMed: | Mannich bases of 4-phenyl-3-buten-2-one: a new class of antiherpes agent. |
| PubMed: | Regulation of epididymal glutathione S-transferases: effects of orchidectomy and androgen replacement. |
| PubMed: | Purification of a new glutathione S-transferase (transferase mu) from human liver having high activity with benzo(alpha)pyrene-4,5-oxide. |
| PubMed: | The presence and longitudinal distribution of the glutathione S-transferases in rat epididymis and vas deferens. |
| PubMed: | Stoichiometric model of alpha-cyclodextrin complex formation. |
| PubMed: | Glutathione S-transferases in earthworms (Lumbricidae). |
| PubMed: | Isoelectric focusing of glutathione S-transferases from rat liver and kidney. |
| PubMed: | [Halogenated 4-phenyl-3-buten-2-one Oximes (author's transl)]. |
| PubMed: | Distribution of enzymes that catalyse reactions of glutathione with alpha beta-unsaturated compounds. |
| PubMed: | Enzymes catalysing conjugations of glutathione with alpha-beta-unsaturated carbonyl compounds. |
|
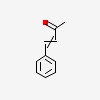 3D/inchi
3D/inchi













