Articles:
4-methyl-2-pentanone
Notes:
Present in orange, lemon, concord grape, vinegar, cheeses, cooked beef, roasted peanut and other foodstuffs. Flavouring ingredient
Methyl isobutyl ketone (MIBK) is an organic solvent. MIBK is among the top ten most popular organic solvents used in industry. MIBK is occasionally found as a volatile component of urine. MIBK in urine is considered as a biological marker of occupational exposure to this solvent. Olfactory perception is significant but adaptation may occur. The typical toxicity effects of MIBK in humans exposed at 50 to 100 ppm are mucous membrane irritation and weak effects on the central nervous system (CNS) such as headache. Visual dysfunction has been reported in workers exposed to a mixture of organic solvents containing MIBK. Memory impairment was detected in clinical observation on a 44-year-old man who had been exposed to MIBK at 100 ppm for more than 10 years. Regarding to the route of absorption, skin penetration of MIBK is substantial. (PMID: 12592578, 17485256, 16464817, 5556886)
| CAS Number: | 108-10-1 | 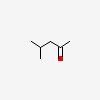 3D/inchi 3D/inchi
|
| Other(deleted CASRN): | 1338234-91-5 | |
| ECHA EINECS - REACH Pre-Reg: | 203-550-1 | |
| FDA UNII: | U5T7B88CNP | |
| Nikkaji Web: | J2.861D | |
| Beilstein Number: | 0605399 | |
| MDL: | MFCD00008938 | |
| CoE Number: | 151 | |
| XlogP3: | 1.30 (est) | |
| Molecular Weight: | 100.16084000 | |
| Formula: | C6 H12 O | |
| BioActivity Summary: | listing | |
| NMR Predictor: | Predict (works with chrome or firefox) | |
Category: cosmetic, flavor and fragrance agents
US / EU / FDA / JECFA / FEMA / FLAVIS / Scholar / Patent Information:
Physical Properties:
| Appearance: | colorless clear liquid (est) |
| Assay: | 99.00 to 100.00 % |
| Food Chemicals Codex Listed: | Yes |
| Specific Gravity: | 0.79600 to 0.79900 @ 25.00 °C. |
| Pounds per Gallon - (est).: | 6.624 to 6.648 |
| Refractive Index: | 1.39100 to 1.39600 @ 20.00 °C. |
| Melting Point: | -84.00 °C. @ 760.00 mm Hg |
| Boiling Point: | 116.50 °C. @ 760.00 mm Hg |
| Acid Value: | 2.00 max. KOH/g |
| Vapor Pressure: | 19.900000 mmHg @ 25.00 °C. |
| Vapor Density: | 3.5 ( Air = 1 ) |
| Flash Point: | 56.00 °F. TCC ( 13.33 °C. ) |
| logP (o/w): | 1.310 |
| Soluble in: | |
| alcohol | |
| dipropylene glycol | |
| water, 1.90E+04 mg/L @ 25 °C (exp) | |
Organoleptic Properties:
| Odor Type: green | |
| sharp solvent green herbal fruity dairy spicy | |
| Odor Description: at 10.00 % in dipropylene glycol. | sharp solvent green herbal fruity dairy spice |
| sharp solvent green herbal fruity dairy | |
| Odor Description: | Sharp solvent-like with green, herbal, fruity and dairy nuances Mosciano, Gerard P&F 21, No. 1, 33, (1996) |
| Flavor Type: green | |
| green vegetable herbal fruity dairy | |
| Taste Description: | Green, vegetative, herbal, fruity and dairy nuances Mosciano, Gerard P&F 21, No. 1, 33, (1996) |
| Odor and/or flavor descriptions from others (if found). | |
| Bedoukian Research | |
| METHYL ISOBUTYL KETONE ≥99.0%, FCC, Kosher, Special Order | |
| Odor Description: | A sharp, green, herbal, fruity, spicy aroma Used for its ethereal quality in fragrances. |
| Taste Description: | green Used in spice, apple, whiskey, fruit and ripe banana flavors. |
| Alfrebro | |
| 4-METHYL-2-PENTANONE NATURAL | |
| Odor Description: | Fruity, Ethereal, Spicy |
| Pell Wall Perfumes | |
| Isobutyl Methyl Ketone | |
| Odor Description: | Sharp, solvent-like, ethereal, fruity, green. Diffusive Used to give an ethereal quality to fragrances, add ‘lift’ and is particularly useful where a fruity element is desired, where it can enhance the top note, giving a more juicy effect. Usage level is normally well below 1% of the fragrance concentrate. |
Cosmetic Information:
| CosIng: | cosmetic data |
| Cosmetic Uses: |
denaturants perfuming agents solvents |
Suppliers:
| Alfrebro |
| 4-METHYL-2-PENTANONE NATURAL
Odor: Fruity, Ethereal, Spicy |
| Arkema |
| Methylisobutylketone
Odor: characteristic Use: MIBK (Methylisobutylketone) is a stable, medium evaporating liquid and compatible with many organic materials, which makes it a good industrial solvent. MIBK can also be used in order to extract, separate and purify various products. It is used in the pharmaceutical, agrochemical industries, in fine chemistry… |
| Eastman Chemical |
| Eastman™ Methyl Isobutyl Ketone
Odor: characteristic Use: Eastman™ Methyl Isobutyl Ketone (MIBK) is a medium-evaporating solvent. It is an active solvent for many synthetic resins including cellulosics, vinyl copolymers, acrylics, alkyds, polyesters, and epoxies. It is very useful in developing high-solids coatings because of its combination of high solvent activity and low density.
In addition to its use as a solvent for inks, coatings, and adhesives, MIBK is used as an extraction agent in the dewaxing and deoiling of petroleum products. It is also used in the manufacture of germicides, fungicides, pharmaceuticals, electroplating solutions, and as a denaturant in many ethanol formulations. |
| ECSA Chemicals |
| METHYL ISOBUTYL KETONE RPE-ACS-FOR ANALYSIS- |
| ECSA TRADE THE MOST UPDATED FINANCIAL PUBLICATION ON THE WORLD OF CHEMISTRY |
| ECSA Chemicals |
| METHYL ISOBUTYL KETONE |
| EMD Millipore |
| For experimental / research use only. |
| isoButyl Methyl Ketone |
| Glentham Life Sciences |
| 4-Methyl-2-pentanone |
| Inoue Perfumery |
| isoBUTYL METHYL KETONE |
| Moellhausen |
| METHYL ISOBUTYL KETONE
Odor: fresh, fruity, somewhat burnt; banana-like with dairy nuance |
| Pell Wall Perfumes |
| Isobutyl Methyl Ketone
Odor: Sharp, solvent-like, ethereal, fruity, green. Diffusive Use: Used to give an ethereal quality to fragrances, add ‘lift’ and is particularly useful where a fruity element is desired, where it can enhance the top note, giving a more juicy effect. Usage level is normally well below 1% of the fragrance concentrate. |
| Penta International |
| METHYL ISOBUTYL KETONE NATURAL |
| Penta International |
| METHYL ISOBUTYL KETONE |
| Shiva Chemicals and Pharmaceuticals |
| Methyl Iso Butyl Ketone (MIBK) |
| Sigma-Aldrich |
| 4-Methyl-2-pentanone, ≥99%, FCC
Odor: spicy; ethereal; fruity |
| Certified Food Grade Products |
| Silver Fern Chemical |
| Methyl Isobutyl Ketone (MIBK)
Odor: characteristic Use: Methyl isobutyl ketone is primarily used as a solvent for paints, varnishes, nitrocellulose, lacquers, organic synthesis, and a denaturant for alcohol. |
| TCI AMERICA |
| For experimental / research use only. |
| 4-Methyl-2-pentanone >99.5%(GC) |
| Wedor Corporation |
| METHYL ISOBUTYL KETONE |
Safety Information:
| Preferred SDS: View | |
| European information : | |
| Most important hazard(s): | |
| Xn - Harmful. | |
|
R 11 - Highly flammable. R 20 - Harmful by inhalation. R 36/37 - Irritating to eyes and respiratory system. R 66 - Repeated exposure may cause skin dryness or cracking. S 02 - Keep out of the reach of children. S 09 - Keep container in a well-ventilated place. S 16 - Keep away from sources of ignition - No Smoking. S 29 - Do not empty into drains. | |
| Hazards identification | |
| Classification of the substance or mixture | |
| GHS Classification in accordance with 29 CFR 1910 (OSHA HCS) | |
| None found. | |
| GHS Label elements, including precautionary statements | |
| Pictogram | |
| Hazard statement(s) | |
| None found. | |
| Precautionary statement(s) | |
| None found. | |
| Oral/Parenteral Toxicity: | |
|
oral-rat LD50 2080 mg/kg (Smyth et al., 1951) oral-mouse LD50 [sex: M] 2670 mg/kg (Tanii et al., 1986) oral-mouse LD50 1200 mg/kg (McOmie & Anderson, 1949a) intraperitoneal-guinea pig LD50 800 mg/kg "Patty's Industrial Hygiene and Toxicology," 3rd rev. ed., Clayton, G.D., and F.E. Clayton, eds., New York, John Wiley & Sons, Inc., 1978-82. Vol. 3 originally pub. in 1979; pub. as 2n rev. ed. in 1985.Vol. 2C, Pg. 4748, 1982. oral-guinea pig LD50 1600 mg/kg "Patty's Industrial Hygiene and Toxicology," 3rd rev. ed., Clayton, G.D., and F.E. Clayton, eds., New York, John Wiley & Sons, Inc., 1978-82. Vol. 3 originally pub. in 1979; pub. as 2n rev. ed. in 1985.Vol. 2C, Pg. 4748, 1982. unreported-mammal (species unspecified) LD50 1396 mg/kg Gigiena i Sanitariya. For English translation, see HYSAAV. Vol. 51(5), Pg. 61, 1986. intraperitoneal-mouse LD50 268 mg/kg Shell Chemical Company. Unpublished Report. Vol. -, Pg. 7, 1961. oral-mouse LD50 1900 mg/kg National Technical Information Service. Vol. OTS0535383 intraperitoneal-rat LD50 400 mg/kg "Patty's Industrial Hygiene and Toxicology," 3rd rev. ed., Clayton, G.D., and F.E. Clayton, eds., New York, John Wiley & Sons, Inc., 1978-82. Vol. 3 originally pub. in 1979; pub. as 2n rev. ed. in 1985.Vol. 2C, Pg. 4748, 1982. oral-rat LD50 2080 mg/kg Union Carbide Data Sheet. Vol. 4/25/1958 | |
| Dermal Toxicity: | |
|
skin-rabbit LD50 > 3000 mg/kg National Technical Information Service. Vol. OTS0535383 | |
| Inhalation Toxicity: | |
|
inhalation-rat LC50 100000 mg/m3 National Technical Information Service. Vol. OTS0535383 inhalation-mouse LC50 23300 mg/m3 Gigiena Truda i Professional'nye Zabolevaniya. Labor Hygiene and Occupational Diseases. Vol. 17(11), Pg. 52, 1973. inhalation-guinea pig LCLo 68900 mg/m3/3H LUNGS, THORAX, OR RESPIRATION: OTHER CHANGES CARDIAC: PULSE RATE SENSE ORGANS AND SPECIAL SENSES: OTHER: EYE National Technical Information Service. Vol. OTS0535383 inhalation-man TCLo 2290 mg/m3 SENSE ORGANS AND SPECIAL SENSES: OTHER CHANGES: OLFACTION National Technical Information Service. Vol. OTS0535383 | |
Safety in Use Information:
| Category: | cosmetic, flavor and fragrance agents | ||
| RIFM Fragrance Material Safety Assessment: Search | |||
| IFRA Code of Practice Notification of the 49th Amendment to the IFRA Code of Practice | |||
| Recommendation for isobutyl methyl ketone usage levels up to: | |||
| 0.5000 % in the fragrance concentrate. | |||
| Maximised Survey-derived Daily Intakes (MSDI-EU): | 6.10 (μg/capita/day) | ||
| Use levels for FEMA GRAS flavoring substances on which the FEMA Expert Panel based its judgments that the substances are generally recognized as safe (GRAS). | |||
| The Expert Panel also publishes separate extensive reviews of scientific information on all FEMA GRAS flavoring substances and can be found at FEMA Flavor Ingredient Library | |||
| publication number: 3 | |||
| Click here to view publication 3 | |||
| average usual ppm | average maximum ppm | ||
| baked goods: | - | 6.30000 | |
| beverages(nonalcoholic): | - | 6.30000 | |
| beverages(alcoholic): | - | - | |
| breakfast cereal: | - | - | |
| cheese: | - | - | |
| chewing gum: | - | - | |
| condiments / relishes: | - | - | |
| confectionery froastings: | - | - | |
| egg products: | - | - | |
| fats / oils: | - | - | |
| fish products: | - | - | |
| frozen dairy: | - | 6.30000 | |
| fruit ices: | - | 6.30000 | |
| gelatins / puddings: | - | - | |
| granulated sugar: | - | - | |
| gravies: | - | - | |
| hard candy: | - | 6.30000 | |
| imitation dairy: | - | - | |
| instant coffee / tea: | - | - | |
| jams / jellies: | - | - | |
| meat products: | - | - | |
| milk products: | - | - | |
| nut products: | - | - | |
| other grains: | - | - | |
| poultry: | - | - | |
| processed fruits: | - | - | |
| processed vegetables: | - | - | |
| reconstituted vegetables: | - | - | |
| seasonings / flavors: | - | - | |
| snack foods: | - | - | |
| soft candy: | - | - | |
| soups: | - | - | |
| sugar substitutes: | - | - | |
| sweet sauces: | - | - | |
Safety References:
| European Food Safety Athority(EFSA): | Flavor usage levels; Subacute, Subchronic, Chronic and Carcinogenicity Studies; Developmental / Reproductive Toxicity Studies; Genotoxicity Studies... |
| European Food Safety Authority (EFSA) reference(s): | |
| Opinion of the Scientific Panel on food additives, flavourings, processing aids and materials in contact with food (AFC) related to Flavouring Group Evaluation 7 (FGE.07): Saturated and unsaturated aliphatic secondary alcohols, ketones and esters of secondary alcohols and saturated linear or branched-chain carboxylic acids from chemical group 5 View page or View pdf | |
| Flavouring Group Evaluation 63 (FGE.63): Consideration of aliphatic secondary alcohols, ketones and related esters evaluated by JECFA (59th meeting) structurally related to saturated and unsaturated aliphatic secondary alcohols, ketones and esters of secondary alcohols and saturated linear or branched-chain carboxylic acids evaluated by EFSA in FGE.07 (2005) (Commission Regulation (EC) No 1565/2000 of 18 July 2000) View page or View pdf | |
| Flavouring Group Evaluation 7, Revision 1 (FGE.07Rev1): Saturated and unsaturated aliphatic secondary alcohols, ketones and esters of secondary alcohols and saturated linear or branched-chain carboxylic acids from chemical group 5 (Commission Regulation (EC) No 1565/2000 of 18 July 2000) - Scientific opinion of the Panel on Food Additives, Flavourings, Processing Aids and Materials in contact with Food (AFC) on a request from the Commission View page or View pdf | |
| Flavouring Group Evaluation 7, Revision 2 (FGE.07Rev2) : Saturated and unsaturated aliphatic secondary alcohols, ketones and esters of secondary alcohols and saturated linear or branched-chain carboxylic acids from chemical group 5 View page or View pdf | |
| EPI System: | View |
| EPA-Iris: | IRIS |
| NIOSH International Chemical Safety Cards: | search |
| NIOSH Pocket Guide: | search |
| Chemical Carcinogenesis Research Information System: | Search |
| AIDS Citations: | Search |
| Cancer Citations: | Search |
| Toxicology Citations: | Search |
| EPA Substance Registry Services (TSCA): | 108-10-1 |
| EPA ACToR: | Toxicology Data |
| EPA Substance Registry Services (SRS): | Registry |
| Laboratory Chemical Safety Summary : | 7909 |
| National Institute of Allergy and Infectious Diseases: | Data |
| WISER: | UN 1245 |
| WGK Germany: | 1 |
| 4-methylpentan-2-one | |
| Chemidplus: | 0000108101 |
| EPA/NOAA CAMEO: | hazardous materials |
| RTECS: | 108-10-1 |
References:
| 4-methylpentan-2-one | |
| NIST Chemistry WebBook: | Search Inchi |
| Canada Domestic Sub. List: | 108-10-1 |
| Pubchem (cid): | 7909 |
| Pubchem (sid): | 134972890 |
| Pherobase: | View |
Other Information:
| (IUPAC): | Atomic Weights of the Elements 2011 (pdf) |
| Videos: | The Periodic Table of Videos |
| tgsc: | Atomic Weights use for this web site |
| (IUPAC): | Periodic Table of the Elements |
| FDA Substances Added to Food (formerly EAFUS): | View |
| FDA Indirect Additives used in Food Contact Substances: | View |
| CHEBI: | View |
| CHEMBL: | View |
| KEGG (GenomeNet): | C19263 |
| HMDB (The Human Metabolome Database): | HMDB02939 |
| FooDB: | FDB008174 |
| Export Tariff Code: | 2914.19.0000 |
| Typical G.C. | |
| VCF-Online: | VCF Volatile Compounds in Food |
| ChemSpider: | View |
| Wikipedia: | View |
Potential Blenders and core components note
Potential Uses:
| apple | FR | |
| banana | FR | |
| cherry | FR | |
| cream | FR | |
| dairy | FL | |
| fruit | FR | |
| grape | FR | |
| green | FR | |
| herbal | FR | |
| mint | FR | |
| orange | FR | |
| pear | FR | |
| pineapple | FR | |
| spice | FR | |
| whiskey | FL |
Occurrence (nature, food, other): note
| beer Search PMC Picture | |
| coffee Search PMC Picture | |
| elder black elder flower oil Search Trop Picture | |
| ginger oil Search Trop Picture | |
| grape Search Trop Picture | |
| lavender oil spike spain @ 0.003% Data GC Search Trop Picture | |
| orange fruit Search Trop Picture | |
| strawberry fruit 0.40% Search Trop Picture | |
| tagete oil rwanda @ 0.03% Data GC Search Trop Picture | |
| vinegar Search Picture |
Synonyms:
| iso | butylmethyl ketone |
| iso | hexanone |
| hexone | |
| ketone, isobutyl methyl | |
| methyl 2-methylpropyl ketone | |
| 4- | methyl 2-pentanone |
| methyl iso-butyl ketone | |
| methyl isobutyl ketone | |
| methyl isobutyl ketone natural | |
| 4- | methyl pentan-2-one |
| 2- | methyl propyl methyl ketone |
| 4- | methyl-2-oxopentane |
| 4- | methyl-2-pentanone |
| 2- | methyl-4-oxopentane |
| 2- | methyl-4-pentanone |
| methyl-isobutylketone | |
| 4- | methyl-pentan-2-one |
| methylisobutylketone | |
| 4- | methylpentan-2-one |
| 2- | methylpropyl methyl ketone |
| mibk | |
| 2- | pentanone, 4-methyl- |
| iso | propyl acetone |







