|
Category: flavor and fragrance agents
US / EU / FDA / JECFA / FEMA / FLAVIS / Scholar / Patent Information:
Physical Properties:
| Appearance: | colorless to pale yellow clear liquid (est) |
| Assay: | 92.00 to 100.00 % sum of isomers
|
| Food Chemicals Codex Listed: | Yes |
| Specific Gravity: | 0.83500 to 0.84500 @ 25.00 °C.
|
| Pounds per Gallon - (est).: | 6.948 to 7.031
|
| Refractive Index: | 1.44900 to 1.45500 @ 20.00 °C.
|
| Boiling Point: | 84.00 to 86.00 °C. @ 19.00 mm Hg
|
| Boiling Point: | 50.00 to 55.00 °C. @ 2.50 mm Hg
|
| Acid Value: | 5.00 max. KOH/g
|
| Vapor Pressure: | 0.552000 mmHg @ 25.00 °C. (est) |
| Vapor Density: | >1 ( Air = 1 ) |
| Flash Point: | 155.00 °F. TCC ( 68.33 °C. )
|
| logP (o/w): | 2.809 (est) |
| Soluble in: |
| | alcohol | | | fixed oils | | | water, 612.7 mg/L @ 25 °C (est) |
Organoleptic Properties:
| |
| Odor Type: fatty |
| |
| Odor Strength: | high ,
recommend smelling in a 1.00 % solution or less |
| |
| Substantivity: | 68 hour(s) at 100.00 % |
| |
| | fresh cucumber fatty green herbal banana waxy green leafy |
Odor Description:
at 1.00 % in dipropylene glycol. | fresh cucumber fatty green herbal banana waxy green leaf
Luebke, William tgsc, (1988) |
| |
| |
| Flavor Type: fatty |
| |
| | sweet green citrus peel spicy cucumber oily fatty brothy |
Taste Description:
| sweet green citrus peel spicy cucumber oily fatty brothy
Luebke, William tgsc, (1988) |
| |
| Odor and/or flavor descriptions from others (if found). |
| |
| Bedoukian Research |
| trans-2-OCTEN-1-AL ≥96.0% (trans), FCC, Kosher |
| Odor Description: | green, pungent, spicy, vegetable
Adds fresh cucumber, green herbal, and banana topnotes. A great modifier for florals. |
| Taste Description: | Sweet, fatty, citrus peel
Citrus especially orange, fatty notes of nuts especially hazelnut. |
| |
| FCI SAS |
| TRANS-2-OCTENAL |
| Odor Description: | Distinctive green, leafy |
| Taste Description: | Sweet, green |
| |
| |
Cosmetic Information:
Suppliers:
| Advanced Biotech |
| TRANS 2 OCTENAL NATURAL
|
| Alfrebro |
| trans-2-OCTENAL NATURAL 10% IN ETHYL ACETATE
Odor: Fatty Green |
| Alfrebro |
| trans-2-OCTENAL NATURAL 10% IN MCTG
|
| Alfrebro |
| trans-2-OCTENAL NATURAL 10% IN PG
|
| Apple Flavor & Fragrance |
| trans-2-Octen-1-al
|
| Bedoukian Research |
| trans-2-OCTEN-1-AL FCC, NO ANTIOXIDANT
≥96.0% (sum of isomers), Special Order Odor: green, pungent, spicy, vegetable Use: Adds fresh cucumber, green herbal, and banana topnotes. A great modifier for florals. Flavor: Sweet, fatty, citrus peel Citrus especially orange, fatty notes of nuts especially hazelnut. |
| Bedoukian Research |
| trans-2-OCTEN-1-AL
≥96.0% (trans), FCC, Kosher Odor: green, pungent, spicy, vegetable Use: Adds fresh cucumber, green herbal, and banana topnotes. A great modifier for florals. Flavor: Sweet, fatty, citrus peel Citrus especially orange, fatty notes of nuts especially hazelnut. |
| BOC Sciences |
| For experimental / research use only. |
| trans-2-OCTEN-1-AL FCC 97.0% (sum of isomers)
|
| Citrus and Allied Essences |
| trans-2-Octenal
|
| Market Report |
| CJ Latta & Associates |
| TRANS-2-OCTENAL
|
| EGNO Chimie |
| TRANS 2 OCTENAL
|
| FCI SAS |
| TRANS-2-OCTENAL
Odor: Distinctive green, leafy Flavor: Sweet, green |
| Inoue Perfumery |
| TRANS-2-OCTENAL
|
| Jinan Enlighten Chemical Technology(Wutong Aroma ) |
| trans-2-Octenal, Kosherk
|
| Kingchem Laboratories |
| T2 OCTENAL
|
| Lluch Essence |
| TRANS-2-OCTENAL
|
| M&U International |
| Trans-2-Octen-1-AL
|
| Penta International |
| TRANS-2-OCTENAL NATURAL 5% IN ETHYL ALCOHOL
|
| Penta International |
| TRANS-2-OCTENAL NATURAL
|
| Penta International |
| TRANS-2-OCTENAL
|
| Reincke & Fichtner |
| trans-2-Octenal
|
| Santa Cruz Biotechnology |
| For experimental / research use only. |
| trans-2-Octenal 95%
|
| Shanghai Vigen Fine Chemical |
| trans-2-Octenal
|
| Sigma-Aldrich |
| trans-2-Octenal, ≥95%, stabilized, FG
Odor: green; herbaceous; spicy |
| Certified Food Grade Products |
| Synerzine |
| 2-Octenal (trans-)
|
| Taytonn ASCC |
| Trans-2-Octenal
Odor: Cucumber, Fatty, Fresh, Green, Waxy |
| TCI AMERICA |
| For experimental / research use only. |
| trans-2-Octenal >96.0%(GC)
|
| Tengzhou Jitian Aroma Chemiclal |
| trans-2-Octenal
|
Safety Information:
| Preferred SDS: View |
| European information : |
| Most important hazard(s): | | Xi - Irritant |
R 36/38 - Irritating to skin and eyes.
S 02 - Keep out of the reach of children.
S 24/25 - Avoid contact with skin and eyes.
S 26 - In case of contact with eyes, rinse immediately with plenty of water and seek medical advice.
S 37/39 - Wear suitable gloves and eye/face protection.
|
| |
| Hazards identification |
| |
| Classification of the substance or mixture |
| GHS Classification in accordance with 29 CFR 1910 (OSHA HCS) |
| None found. |
| GHS Label elements, including precautionary statements |
| |
| Pictogram | |
| |
| Hazard statement(s) |
| None found. |
| Precautionary statement(s) |
| None found. |
| Oral/Parenteral Toxicity: |
|
Not determined
|
| Dermal Toxicity: |
|
Not determined
|
| Inhalation Toxicity: |
|
Not determined
|
Safety in Use Information:
| Category: | flavor and fragrance agents |
| RIFM Fragrance Material Safety Assessment: Search |
| IFRA Code of Practice Notification of the 49th Amendment to the IFRA Code of Practice |
| Recommendation for (E)-2-octenal usage levels up to: | | | 0.1000 % in the fragrance concentrate.
|
| |
| Maximised Survey-derived Daily Intakes (MSDI-EU): | 0.79 (μg/capita/day) |
| Modified Theoretical Added Maximum Daily Intake (mTAMDI): | 2000 (μg/person/day) |
| Threshold of Concern: | 1800 (μg/person/day) |
| Structure Class: | I |
| Use levels for FEMA GRAS flavoring substances on which the FEMA Expert Panel based its judgments that the substances are generally recognized as safe (GRAS). |
| The Expert Panel also publishes separate extensive reviews of scientific information on all FEMA GRAS flavoring substances and can be found at FEMA Flavor Ingredient Library |
| publication number: 4 |
| Click here to view publication 4 |
| | average usual ppm | average maximum ppm |
| baked goods: | - | 1.00000 |
| beverages(nonalcoholic): | - | - |
| beverages(alcoholic): | - | - |
| breakfast cereal: | - | - |
| cheese: | - | - |
| chewing gum: | - | - |
| condiments / relishes: | - | 1.00000 |
| confectionery froastings: | - | - |
| egg products: | - | - |
| fats / oils: | - | - |
| fish products: | - | - |
| frozen dairy: | - | - |
| fruit ices: | - | - |
| gelatins / puddings: | - | - |
| granulated sugar: | - | - |
| gravies: | - | 1.00000 |
| hard candy: | - | 1.00000 |
| imitation dairy: | - | - |
| instant coffee / tea: | - | - |
| jams / jellies: | - | - |
| meat products: | - | 1.00000 |
| milk products: | - | - |
| nut products: | - | - |
| other grains: | - | - |
| poultry: | - | - |
| processed fruits: | - | - |
| processed vegetables: | - | - |
| reconstituted vegetables: | - | - |
| seasonings / flavors: | - | - |
| snack foods: | - | - |
| soft candy: | - | - |
| soups: | - | 1.00000 |
| sugar substitutes: | - | - |
| sweet sauces: | - | - |
| |
| Food categories according to Commission Regulation EC No. 1565/2000 (EC, 2000) in FGE.06 (EFSA, 2002a). According to the Industry the "normal" use is defined as the average of reported usages and "maximum use" is defined as the 95th percentile of reported usages (EFSA, 2002i). |
| Note: mg/kg = 0.001/1000 = 0.000001 = 1/1000000 = ppm. |
| | average usage mg/kg | maximum usage mg/kg |
| Dairy products, excluding products of category 02.0 (01.0): | 5.70000 | 12.00000 |
| Fats and oils, and fat emulsions (type water-in-oil) (02.0): | 1.50000 | 14.25000 |
| Edible ices, including sherbet and sorbet (03.0): | 0.90000 | 2.98000 |
| Processed fruit (04.1): | - | - |
| Processed vegetables (incl. mushrooms & fungi, roots & tubers, pulses and legumes), and nuts & seeds (04.2): | 5.00000 | 5.03000 |
| Confectionery (05.0): | 5.50000 | 14.46000 |
| Chewing gum (05.0): | - | - |
| Cereals and cereal products, incl. flours & starches from roots & tubers, pulses & legumes, excluding bakery (06.0): | 4.80000 | 11.55000 |
| Bakery wares (07.0): | 8.00000 | 19.07000 |
| Meat and meat products, including poultry and game (08.0): | 0.90000 | 2.98000 |
| Fish and fish products, including molluscs, crustaceans and echinoderms (MCE) (09.0): | 0.90000 | 2.98000 |
| Eggs and egg products (10.0): | 0.90000 | 2.98000 |
| Sweeteners, including honey (11.0): | 0.90000 | 2.98000 |
| Salts, spices, soups, sauces, salads, protein products, etc. (12.0): | 2.00000 | 5.00000 |
| Foodstuffs intended for particular nutritional uses (13.0): | - | - |
| Non-alcoholic ("soft") beverages, excl. dairy products (14.1): | 2.00000 | 4.43000 |
| Alcoholic beverages, incl. alcohol-free and low-alcoholic counterparts (14.2): | 1.00000 | 2.00000 |
| Ready-to-eat savouries (15.0): | 2.50000 | 4.50000 |
| Composite foods (e.g. casseroles, meat pies, mincemeat) - foods that could not be placed in categories 01.0 - 15.0 (16.0): | 0.90000 | 2.98000 |
Safety References:
| Flavor & Extract Manufacturers Association (FEMA) reference(s): |
| The FEMA GRAS assessment of alpha,beta-unsaturated aldehydes and related substances used as flavor ingredients. View pdf |
| European Food Safety Athority(EFSA): | Flavor usage levels; Subacute, Subchronic, Chronic and Carcinogenicity Studies; Developmental / Reproductive Toxicity Studies; Genotoxicity Studies... |
| European Food Safety Authority (EFSA) reference(s): |
EFSA Panel on Food Contact Materials, Enzymes, Flavourings and Processing Aids (CEF)
View page or View pdf |
Scientific Opinion on Flavouring Group Evaluation 200, Revision 1 (FGE.200 Rev.1): 74 a,▀-unsaturated aliphatic aldehydes and precursors from chemical subgroup 1.1.1 of FGE.19
View page or View pdf |
Safety and efficacy of 26 compounds belonging to chemical group 3 (a,▀-unsaturated straight-chain and branched-chain aliphatic primary alcohols, aldehydes, acids and esters) when used as flavourings for all animal species and categories
View page or View pdf |
Scientific Opinion on Flavouring Group Evaluation 5, Revision 3 (FGE.05Rev3): Branched- and straight-chain unsaturated aldehydes, dienals, unsaturated and saturated carboxylic acids and related esters with saturated and unsaturated aliphatic alcohols and a phenylacetic acid related ester from chemical groups 1, 2, 3, 5 and 15
View page or View pdf |
| EPI System: | View |
| AIDS Citations: | Search |
| Cancer Citations: | Search |
| Toxicology Citations: | Search |
| EPA Substance Registry Services (TSCA): | 2548-87-0 |
| EPA ACToR: | Toxicology Data |
| EPA Substance Registry Services (SRS): | Registry |
| Laboratory Chemical Safety Summary : | 5283324 |
| National Institute of Allergy and Infectious Diseases: | Data |
| WGK Germany: | 3 |
| | (E)-oct-2-enal |
| Chemidplus: | 0002548870 |
| RTECS: | RH2130000 for cas# 2548-87-0 |
References:
Other Information:
Potential Blenders and core components note
| |
| For Odor |
| No odor group found for these |
| 6- | methyl-2-heptanol | |
| aldehydic |
| | decanal (aldehyde C-10) | FL/FR |
| | dodecanal (aldehyde C-12 lauric) | FL/FR |
| | fresh carbaldehyde | FR |
| 2- | tridecenal | FL/FR |
| 10- | undecenal (aldehyde C-11 undecylenic) | FL/FR |
| animal |
| | methyl (E)-2-octenoate | FL/FR |
| chocolate |
| 2,5- | dimethyl pyrazine | FL/FR |
| citrus |
| | citral diethyl acetal | FL/FR |
| | citral dimethyl acetal | FL/FR |
| (Z)-7- | decenal | FR |
| (E)-4- | decenal | FL/FR |
| | lemongrass oil | FL/FR |
| | petitgrain combava oil | FR |
| | verbena absolute france | FL/FR |
| creamy |
| 3- | heptyl dihydro-5-methyl-2(3H)-furanone | FL/FR |
| cucumber |
| (R)-(-)-2- | octanol | |
| fatty |
| | butyl undecylenate | FL/FR |
| 3- | decen-2-one | FL/FR |
| | methyl (E)-2-hexenoate | FL/FR |
| | methyl 2-hexenoate | FL/FR |
| (Z)-2- | nonenal | CS |
| (E)-2- | nonenal | FL/FR |
| (Z)-2- | octenal | |
| 2- | octenal | FL/FR |
| (Z)- | oleic acid | FL/FR |
| fermented |
| | methyl decanoate | FL/FR |
| floral |
| | allyl anthranilate | FL/FR |
| | citronellyl formate | FL/FR |
| (Z)-3- | hexen-1-yl salicylate | FL/FR |
| alpha- | hexyl cinnamaldehyde | FL/FR |
| | hexyl oxyacetonitrile | |
| | neroli oil bigarde | FL/FR |
| | neryl formate | FL/FR |
| | phenethyl hexanoate | FL/FR |
| | rhodinyl acetate | FL/FR |
| | rose butanoate | FL/FR |
| | styralyl propionate | FL/FR |
| fruity |
| | allyl heptanoate | FL/FR |
| iso | amyl butyrate | FL/FR |
| iso | amyl isobutyrate | FL/FR |
| iso | butyl 2-butenoate | FL/FR |
| iso | butyl isovalerate | FL/FR |
| | citronellyl isobutyrate | FL/FR |
| | cyclohexyl propionate | FL/FR |
| | decyl butyrate | FL/FR |
| alpha,alpha- | dimethyl benzyl isobutyrate | FL/FR |
| | ethyl 3-hexenoate | FL/FR |
| | ethyl hexanoate | FL/FR |
| (E,E)- | ethyl sorbate | FL/FR |
| (E)-2- | hexen-1-ol | FL/FR |
| 2- | hexen-1-ol | FL/FR |
| (E)-3- | hexen-1-yl acetate | FL/FR |
| | methyl 2-methyl butyrate | FL/FR |
| (E)-7- | methyl-3-octen-2-one | FL/FR |
| | neryl isobutyrate | FL/FR |
| 2- | nonanone propylene glycol acetal | FL/FR |
| | prenyl acetate | FL/FR |
| (E)-2- | undecenal | FL/FR |
| green |
| | acetaldehyde butyl phenethyl acetal | FL/FR |
| | acetaldehyde di-(Z)-3-hexen-1-yl acetal | FL/FR |
| | acetaldehyde ethyl phenethyl acetal | FL/FR |
| 3,5,6-neo | cyclocitral | FR |
| | galbanum oil terpeneless | FL/FR |
| | geranium absolute | FL/FR |
| (Z)-3- | hepten-1-yl acetate | FL/FR |
| (Z)-4- | heptenal | FL/FR |
| | heptyl acetate | FL/FR |
| | heptyl cinnamate | FL/FR |
| 3- | hexen-1-ol | FL/FR |
| (Z)-2- | hexen-1-ol | FL/FR |
| (E)-2- | hexen-1-yl acetate | FL/FR |
| (Z)-3- | hexen-1-yl benzoate | FL/FR |
| (Z)-3- | hexen-1-yl lactate | FL/FR |
| (Z)-3- | hexen-1-yl methyl carbonate | FL/FR |
| (Z)-3- | hexen-1-yl pyruvate | FL/FR |
| (E)-2- | hexenal | FL/FR |
| 3- | hexenal | FL/FR |
| | hexoxyacetaldehyde dimethyl acetal | FR |
| | hexyl isobutyrate | FL/FR |
| (Z)- | leaf acetal | FL/FR |
| | leafy acetal | FL/FR |
| | methyl heptine carbonate | FL/FR |
| | methyl octine carbonate | FL/FR |
| | methyl octine carbonate replacer | FR |
| | methyl R-3-acetoxyhexanoate | |
| 3,6- | nonadien-1-ol | FL/FR |
| 2,6- | nonadien-1-ol | FL/FR |
| (E,Z)-2,6- | nonadien-1-ol | FL/FR |
| (E,Z)-3,6- | nonadien-1-ol | FL/FR |
| (Z,Z)-3,6- | nonadien-1-ol | FL/FR |
| (E,Z)-2,6- | nonadien-1-yl acetate | FL/FR |
| (E,Z)-2,6- | nonadienal | FL/FR |
| 2,6- | nonadienal | FL/FR |
| (E,Z)-2,6- | nonadienal diethyl acetal | FL/FR |
| (Z)-2- | nonen-1-ol | FL/FR |
| | octanal dimethyl acetal | FL/FR |
| (E)-2- | octen-1-ol | FL/FR |
| 2- | octen-1-ol | FL/FR |
| (Z)-5- | octen-1-yl propionate | FL/FR |
| | phenethyl tiglate | FL/FR |
| 3- | phenyl propionaldehyde | FL/FR |
| iso | propyl phenyl propionaldehyde | FR |
| | rose leaf absolute (rosa centifolia) | FL/FR |
| | styralyl acetate | FL/FR |
| | thiogeraniol | FL/FR |
| | violet leaf absolute egypt | FL/FR |
| herbal |
| iso | amyl heptanoate | FL/FR |
| sweet | basil absolute | FL/FR |
| | benzyl octanoate | FL/FR |
| | clary sage absolute | FL/FR |
| | coriander oleoresin | FL/FR |
| | dehydroxylinalool oxide | FL/FR |
| (E)-2- | dodecenal | FL/FR |
| | freesia heptanol | FL/FR |
| | herbal heptane | FR |
| (S)-(-)- | menthofuran | |
| (E,Z)-3,5- | octadien-2-one | |
| | rosemary oleoresin | FL/FR |
| alpha- | terpinyl acetate | FL/FR |
| honey |
| | methyl phenyl acetate | FL/FR |
| melon |
| (Z)-6- | nonenal | FL/FR |
| minty |
| iso | pulegyl formate | FL/FR |
| musty |
| ketoiso | phorone | FL/FR |
| pine |
| | plectranthus glandulosus hook f. leaf oil cameroon | FR |
| spicy |
| 4- | carvomenthenol | FL/FR |
| | methyl heptadienone | FL/FR |
| terpenic |
| para- | cymene | FL/FR |
| | frankincense oil | FL/FR |
| waxy |
| iso | amyl decanoate | FL/FR |
| | hexadecanol | FL/FR |
| 2- | methyl heptanoic acid | FL/FR |
| 2,4- | nonadien-1-ol | FL/FR |
| | octanol | FL/FR |
| woody |
| | camphene | FL/FR |
| |
| For Flavor |
| |
| No flavor group found for these |
| | acetaldehyde di-(Z)-3-hexen-1-yl acetal | FL/FR |
| | allyl anthranilate | FL/FR |
| | benzyl octanoate | FL/FR |
| (Z)-6- | decenal | FL |
| (Z)-3- | hepten-1-yl acetate | FL/FR |
| | heptyl cinnamate | FL/FR |
| | hexanal butane-2,3-diol acetal | FL |
| | hexanal octane-1,3-diol acetal | FL |
| 3- | hexen-1-ol | FL/FR |
| 2- | hexenal | FL |
| (E)-2- | hexenal | FL |
| | hexyl oxyacetonitrile | |
| (S)-(-)- | menthofuran | |
| | methyl (E)-2-hexenoate | FL/FR |
| | methyl 2-hexenoate | FL/FR |
| 3- | methyl-3-pentanol | FL |
| 2,6- | nonadienal | FL/FR |
| 2- | nonanone propylene glycol acetal | FL/FR |
| 2,4,6- | nonatrienal | FL |
| (E,Z)-3,5- | octadien-2-one | |
| (R)-(-)-2- | octanol | |
| (Z)-2- | octenal | |
| 2- | octenal | FL/FR |
| iso | pulegyl formate | FL/FR |
| 3,5- | undecadien-2-one | FL |
| aldehydic |
| 2- | tridecenal | FL/FR |
| apple |
| (E,Z)-2,6- | nonadien-1-ol | FL/FR |
| aromatic |
| | leafy acetal | FL/FR |
| camphoreous |
| | camphene | FL/FR |
| citrus |
| | citral diethyl acetal | FL/FR |
| | citral dimethyl acetal | FL/FR |
| | freesia heptanol | FL/FR |
| | lemongrass oil | FL/FR |
| ketoiso | phorone | FL/FR |
| | styralyl propionate | FL/FR |
| | verbena absolute france | FL/FR |
| cooling |
| 4- | carvomenthenol | FL/FR |
| creamy |
| 2,4- | heptadienal | FL |
| cucumber |
| 2- | ethyl octine carbonate | FL |
| fatty |
| (E,E)-2,4- | decadienal | FL |
| 2,4- | decadienal | FL |
| | diacetyl trimer | FL |
| (E,E)-2,4- | heptadienal | FL |
| (Z)-3- | hexen-1-yl benzoate | FL/FR |
| | methyl decanoate | FL/FR |
| 1- | methyl thio-3-octanone | FL |
| (E)-7- | methyl-3-octen-2-one | FL/FR |
| 2,4- | nonadien-1-ol | FL/FR |
| 2,4- | nonadienal | FL |
| (Z)-2- | nonen-1-ol | FL/FR |
| 2,4- | octadien-1-ol | FL |
| (E)-2- | octen-1-ol | FL/FR |
| 2- | octen-1-ol | FL/FR |
| (Z)- | oleic acid | FL/FR |
| 10- | undecenal (aldehyde C-11 undecylenic) | FL/FR |
| floral |
| | methyl phenyl acetate | FL/FR |
| | neroli oil bigarde | FL/FR |
| | rhodinyl acetate | FL/FR |
| fruity |
| | allyl heptanoate | FL/FR |
| iso | amyl isobutyrate | FL/FR |
| | citronellyl formate | FL/FR |
| | citronellyl isobutyrate | FL/FR |
| | cyclohexyl propionate | FL/FR |
| | decyl butyrate | FL/FR |
| alpha,alpha- | dimethyl benzyl isobutyrate | FL/FR |
| | ethyl 3-hexenoate | FL/FR |
| | ethyl 3-oxohexanoate | FL |
| | ethyl hexanoate | FL/FR |
| (E,E)- | ethyl sorbate | FL/FR |
| | furfuryl propionate | FL |
| 3- | heptyl dihydro-5-methyl-2(3H)-furanone | FL/FR |
| 2- | hexen-1-ol | FL/FR |
| (E)-3- | hexen-1-yl acetate | FL/FR |
| | methyl (E)-2-octenoate | FL/FR |
| | methyl 2-methyl butyrate | FL/FR |
| | neryl formate | FL/FR |
| | neryl isobutyrate | FL/FR |
| | prenyl acetate | FL/FR |
| | rose butanoate | FL/FR |
| | styralyl acetate | FL/FR |
| green |
| | acetaldehyde butyl phenethyl acetal | FL/FR |
| | acetaldehyde ethyl phenethyl acetal | FL/FR |
| iso | butyl 2-butenoate | FL/FR |
| iso | butyl isovalerate | FL/FR |
| | cucumber distillates | FL |
| 3- | decen-2-one | FL/FR |
| (E)-4- | decenal | FL/FR |
| | galbanum oil terpeneless | FL/FR |
| | geranium absolute | FL/FR |
| (Z)-4- | heptenal | FL/FR |
| (E)-2- | heptenal | FL |
| | heptyl acetate | FL/FR |
| (Z)-2- | hexen-1-ol | FL/FR |
| (E)-2- | hexen-1-ol | FL/FR |
| (E)-2- | hexen-1-yl acetate | FL/FR |
| (Z)-3- | hexen-1-yl lactate | FL/FR |
| (Z)-3- | hexen-1-yl methyl carbonate | FL/FR |
| (Z)-3- | hexen-1-yl pyruvate | FL/FR |
| (Z)-3- | hexen-1-yl salicylate | FL/FR |
| 3- | hexenal | FL/FR |
| (E)-2- | hexenal | FL/FR |
| | hexyl isobutyrate | FL/FR |
| (Z)- | leaf acetal | FL/FR |
| | methyl 2-undecynoate | FL |
| | methyl heptadienone | FL/FR |
| | methyl heptine carbonate | FL/FR |
| | methyl octine carbonate | FL/FR |
| | methyl R-3-acetoxyhexanoate | |
| 3,6- | nonadien-1-ol | FL/FR |
| 2,6- | nonadien-1-ol | FL/FR |
| (E,Z)-3,6- | nonadien-1-ol | FL/FR |
| (E,Z)-2,6- | nonadien-1-yl acetate | FL/FR |
| (E,E)-2,6- | nonadienal | FL |
| (E,Z)-2,6- | nonadienal | FL/FR |
| (E,Z)-2,6- | nonadienal diethyl acetal | FL/FR |
| (Z)-6- | nonenal | FL/FR |
| (E)-2- | nonenal | FL/FR |
| 2,4- | octadienal | FL |
| (E,E)-2,4- | octadienal | FL |
| | octanal dimethyl acetal | FL/FR |
| (Z)-5- | octen-1-yl propionate | FL/FR |
| | phenethyl tiglate | FL/FR |
| 3- | phenyl propionaldehyde | FL/FR |
| | rose leaf absolute (rosa centifolia) | FL/FR |
| | thiogeraniol | FL/FR |
| | violet leaf absolute egypt | FL/FR |
| herbal |
| iso | amyl heptanoate | FL/FR |
| sweet | basil absolute | FL/FR |
| | clary sage absolute | FL/FR |
| | coriander oleoresin | FL/FR |
| | rosemary oleoresin | FL/FR |
| mushroom |
| | methional diethyl acetal | FL |
| musty |
| 2,5- | dimethyl pyrazine | FL/FR |
| soapy |
| | dodecanal (aldehyde C-12 lauric) | FL/FR |
| sweet |
| | acetone alcohol | FL |
| terpenic |
| para- | cymene | FL/FR |
| waxy |
| iso | amyl butyrate | FL/FR |
| iso | amyl decanoate | FL/FR |
| | butyl undecylenate | FL/FR |
| | decanal (aldehyde C-10) | FL/FR |
| (E)-2- | dodecenal | FL/FR |
| | furfuryl octanoate | FL |
| | hexadecanol | FL/FR |
| alpha- | hexyl cinnamaldehyde | FL/FR |
| 2- | methyl heptanoic acid | FL/FR |
| 6- | methyl-2-heptanol | |
| (Z,Z)-3,6- | nonadien-1-ol | FL/FR |
| | octanol | FL/FR |
| | phenethyl hexanoate | FL/FR |
| (E)-2- | undecenal | FL/FR |
| woody |
| | dehydroxylinalool oxide | FL/FR |
| | frankincense oil | FL/FR |
| alpha- | terpinyl acetate | FL/FR |
| |
Potential Uses:
Occurrence (nature, food, other): noteSynonyms:
| (2E)- | oct-2-enal | | (E)- | oct-2-enal | | trans- | oct-2-enal | | (E)-2- | octen-1-al | | trans-2- | octen-1-al | | trans-2- | octen-1-al FCC, no antioxidant | | trans- | octen-2-al | | (2E)-2- | octenal | | (E)-2- | octenal | | 2-trans- | octenal | | T2 | octenal | | trans-2- | octenal | | 2- | octenal (trans-) | | 2- | octenal, (2E)- | | 2- | octenal, (E)- | | trans-2- | octenal, natural |
Articles:
| Info: | Volatile Flavor Components in Bogyojosaeng and Suhong Cultivars of Strawberry (Fragaria ananassa Duch.) |
| PubMed: | Characterization of the Key Odorants in Chinese Chixiang Aroma Type Liquor by Gas Chromatography-Olfactometry, Quantitative Measurements, Aroma Recombination, and Omission Studies. |
| PubMed: | Bed bug aggregation pheromone finally identified. |
| PubMed: | Reactions of the selected ion flow tube mass spectrometry reagent ions H3O(+) and NO(+) with a series of volatile aldehydes of biogenic significance. |
| PubMed: | Theoretical study of the gas-phase reactions of NO3 radical with a series of trans-2-unsaturated aldehydes: from acrolein to trans-2-octenal. |
| PubMed: | Exocrine secretions of wheel bugs (Heteroptera: Reduviidae: Arilus spp.): clarification and chemistry. |
| PubMed: | The effects of fungal volatile organic compounds on bone marrow stromal cells. |
| PubMed: | Ultrahigh performance liquid chromatography analysis of volatile carbonyl compounds in virgin olive oils. |
| PubMed: | Identification of volatiles from oxidised phosphatidylcholine molecular species using headspace solid-phase microextraction (HS-SPME) and gas chromatography-mass spectrometry (GC-MS). |
| PubMed: | Effects of aliphatic aldehydes on the growth and patulin production of Penicillium expansum in apple juice. |
| PubMed: | Identification of characteristic flavour precursors from enzymatic hydrolysis-mild thermal oxidation tallow by descriptive sensory analysis and gas chromatography-olfactometry and partial least squares regression. |
| PubMed: | Shelf-life of infrared dry-roasted almonds. |
| PubMed: | Real-time measurement of volatile chemicals released by bed bugs during mating activities. |
| PubMed: | Defensive roles of (E)-2-alkenals and related compounds in heteroptera. |
| PubMed: | Antibacterial activity of 4-oxo-(E)-2-hexenal from adults and nymphs of the heteropteran, Dolycoris baccarum (Heteroptera: Pentatomidae). |
| PubMed: | Toxicity and metabolism of exogenous ╬▒,╬▓-unsaturated carbonyls in potato (Solanum tuberosum L.) tubers. |
| PubMed: | An experimental study of the gas-phase reactions of NO3 radicals with a series of unsaturated aldehydes: trans-2-hexenal, trans-2-heptenal, and trans-2-octenal. |
| PubMed: | Iron-lactoferrin complex reduces iron-catalyzed off-flavor formation in powdered milk with added fish oil. |
| PubMed: | Differences in the volatile compositions of ginseng species (Panax sp.). |
| PubMed: | Gas chromatographic-olfactometric aroma profile and quantitative analysis of volatile carbonyls of grilled beef from different finishing feed systems. |
| PubMed: | Millipedes that smell like bugs: (E)-alkenals in the defensive secretion of the julid diplopod Allajulus dicentrus. |
| PubMed: | Change of volatile compounds in fresh fish meat during ice storage. |
| PubMed: | Simultaneous sampling and analysis of indoor air infested with Cimex lectularius L. (Hemiptera: Cimicidae) by solid phase microextraction, thin film microextraction and needle trap device. |
| PubMed: | Effect of enzyme activity and frozen storage on jalape├▒o pepper volatiles by selected ion flow tube-mass spectrometry. |
| PubMed: | Alarm pheromones and chemical communication in nymphs of the tropical bed bug Cimex hemipterus (Hemiptera: Cimicidae). |
| PubMed: | A new method for the determination of carbonyl compounds in wines by headspace solid-phase microextraction coupled to gas chromatography-ion trap mass spectrometry. |
| PubMed: | Nymphs of the common bed bug (Cimex lectularius) produce anti-aphrodisiac defence against conspecific males. |
| PubMed: | Molecular characterization, expression analysis, and role of ALDH3B1 in the cellular protection against oxidative stress. |
| PubMed: | Neurotoxicity of fungal volatile organic compounds in Drosophila melanogaster. |
| PubMed: | Comparison of tomatillo and tomato volatile compounds in the headspace by selected ion flow tube mass spectrometry (SIFT-MS). |
| PubMed: | 4-oxo-aldehydes from the dorsal abdominal glands of the bed bug (Hemiptera: Cimicidae). |
| PubMed: | Characterization of the antennal olfactory system of the bed bug (Cimex lectularius). |
| PubMed: | Effect of temperature on lipid-related volatile production in tomato puree. |
| PubMed: | Characterization and quantification of odor-active compounds in unsaturated fatty acid/conjugated linoleic acid (UFA/CLA)-enriched butter and in conventional butter during storage and induced oxidation. |
| PubMed: | Addition of alarm pheromone components improves the effectiveness of desiccant dusts against Cimex lectularius. |
| PubMed: | Volatile composition of Catharanthus roseus (L.) G. Don using solid-phase microextraction and gas chromatography/mass spectrometry. |
| PubMed: | Identification of the airborne aggregation pheromone of the common bed bug, Cimex lectularius. |
| PubMed: | Comparison of odor-active compounds from six distinctly different rice flavor types. |
| PubMed: | Toxic oxygenated alpha,beta-unsaturated aldehydes and their study in foods: a review. |
| PubMed: | Light-induced off-flavor development in cloudy apple juice. |
| PubMed: | Truffle volatiles inhibit growth and induce an oxidative burst in Arabidopsis thaliana. |
| PubMed: | Semiochemical investigations of the insidious flower bug, Orius insidiosus (Say). |
| PubMed: | Inter- and intraspecific variation in defensive compounds produced by five neotropical stink bug species (Hemiptera: Pentatomidae). |
| PubMed: | Instrumental and sensory characterization of heat-induced odorants in aseptically packaged soy milk. |
| PubMed: | An assessment of the role played by some oxidation-related aldehydes in wine aroma. |
| PubMed: | In vitro antifungal and anti-elastase activity of some aliphatic aldehydes from Olea europaea L. fruit. |
| PubMed: | Difference in the volatile composition of pine-mushrooms (Tricholoma matsutake Sing.) according to their grades. |
| PubMed: | Critical aspects of the determination of pentafluorobenzyl derivatives of aldehydes by gas chromatography with electron-capture or mass spectrometric detection: Validation of an optimized strategy for the determination of oxygen-related odor-active aldehydes in wine. |
| PubMed: | Short and simple syntheses of 4-oxo-(E)-2-hexenal and homologs: pheromone components and defensive compounds of Hemiptera. |
| PubMed: | Identification of the larval aggregation pheromone of codling moth, Cydia pomonella. |
| PubMed: | Structural characterization of an etheno-2'-deoxyguanosine adduct modified by tetrahydrofuran. |
| PubMed: | Mastrus ridibundus parasitoids eavesdrop on cocoon-spinning codling moth, Cydia pomonella, larvae. |
| PubMed: | Comparison of the olfactory sensitivity of two sympatric steppe grasshopper species (Orthoptera: Acrididae) to plant volatile compounds. |
| PubMed: | Formation of volatile compounds in model experiments with crude leek (Allium ampeloprasum Var. Lancelot) enzyme extract and linoleic acid or linolenic acid. |
| PubMed: | Volatile components in metatarsal glands of sika deer, Cervus nippon. |
| PubMed: | Semiochemicals from the predatory stink bug Eocanthecona furcellata (Wolff): components of metathoracic gland, dorsal abdominal gland, and sternal gland secretions. |
| PubMed: | Identification of volatile compounds in soybean at various developmental stages using solid phase microextraction. |
| PubMed: | Identification of aroma active compounds in orange essence oil using gas chromatography-olfactometry and gas chromatography-mass spectrometry. |
| PubMed: | Determination of stale-flavor carbonyl compounds in beer by stir bar sorptive extraction with in-situ derivatization and thermal desorption-gas chromatography-mass spectrometry. |
| PubMed: | Kairomone strains of Euclytiaflava (Townsend), a parasitoid of stink bugs. |
| PubMed: | Study on the mechanisms of the antibacterial action of some plant alpha,beta-unsaturated aldehydes. |
| PubMed: | Character impact odorants of the apple cultivars Elstar and Cox Orange. |
| PubMed: | Synthesis, characterization and X-ray structures of new antiproliferative and proapoptotic natural aldehyde thiosemicarbazones and their nickel(II) and copper(II) complexes. |
| PubMed: | Chemical defense in the plant bug Lopidea robiniae (Uhler). |
| PubMed: | 1,N6-etheno-2'-deoxyadenosine adducts from trans, trans-2,4-decadienal and trans-2-octenal. |
| PubMed: | DNA-damaging potential and glutathione depletion of 2-cyclohexene-1-one in mammalian cells, compared to food relevant 2-alkenals. |
| PubMed: | In vitro antibacterial activity of some aliphatic aldehydes from Olea europaea L. |
| PubMed: | Selective protection by stably transfected human ALDH3A1 (but not human ALDH1A1) against toxicity of aliphatic aldehydes in V79 cells. |
| PubMed: | Inhibition of formation of oxidative volatile components in fermented cucumbers by ascorbic acid and turmeric. |
| PubMed: | Aroma of fresh oysters Crassostrea gigas: composition and aroma notes. |
| PubMed: | Novel 1,N(6)-etheno-2'-deoxyadenosine adducts from lipid peroxidation products. |
| PubMed: | Development of oxidized odor and volatile aldehydes in fermented cucumber tissue exposed to oxygen. |
| PubMed: | Influence of variety and growing location on the development of off-flavor in precooked vacuum-packed potatoes. |
| PubMed: | Synthesis of trans-4,5-epoxy-(E)-2-decenal and its deuterated analog used for the development of a sensitive and selective quantification method based on isotope dilution assay with negative chemical ionization. |
| PubMed: | Structural and kinetic determinants of aldehyde reduction by aldose reductase. |
| PubMed: | Changes induced in bovine serum albumin following interactions with the lipid peroxidation product E-2-octenal. |
| PubMed: | Determination of aldehydes and other lipid peroxidation products in biological samples by gas chromatography-mass spectrometry. |
| PubMed: | Alpha,beta-unsaturated carbonyl compounds: inhibition of rat liver glutathione S-transferase isozymes and chemical reaction with reduced glutathione. |
| PubMed: | Modification of delipidated apoprotein B of low density lipoprotein by lipid oxidation products in relation to macrophage scavenger receptor binding. |
| PubMed: | Defensive secretion of rice bug,Leptocorisa oratorius fabricius, (Hemiptera: Coreidae): A unique chemical combination and its toxic, repellent, and alarm properties. |
| PubMed: | Volatile compounds from the predatory insectPodisus maculiventris (Hemiptera: Pentatomidae) : Male and female metathoracic scent gland and female dorsal abdominal gland secretions. |
|
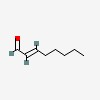 3D/inchi
3D/inchi












