Articles:
p-mentha-1,8-dien-7-al
Notes:
from oil of perillae herba; has neuropharmacological actions. Constit. of Perilla nankinensis and gingergrass oils
Perillaldehyde, or perilla aldehyde, is a natural organic compound found most abundantly in the annual herb perilla, but also in a wide variety of other plants and essential oils. It is a monoterpenoid containing an aldehyde functional group.; Perillaldehyde, or perilla aldehyde, is a natural organic compound found most abundantly in the perennial herb perilla, but also in a wide variety of other plants and essential oils. It is a monoterpenoid containing an aldehyde functional group.
| CAS Number: | 2111-75-3 | 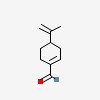 3D/inchi 3D/inchi
|
| Other(deleted CASRN): | 1254961-45-9 | |
| ECHA EINECS - REACH Pre-Reg: | 218-302-8 | |
| FDA UNII: | 6EQL0XA86G | |
| Nikkaji Web: | J2.142C | |
| MDL: | MFCD00001543 | |
| CoE Number: | 11788 | |
| XlogP3-AA: | 2.60 (est) | |
| Molecular Weight: | 150.22078000 | |
| Formula: | C10 H14 O | |
| BioActivity Summary: | listing | |
| NMR Predictor: | Predict (works with chrome or firefox) | |
Category: cosmetic, flavor and fragrance agents
US / EU / FDA / JECFA / FEMA / FLAVIS / Scholar / Patent Information:
| Google Scholar: | Search |
| Google Books: | Search |
| Google Scholar: with word "volatile" | Search |
| Google Scholar: with word "flavor" | Search |
| Google Scholar: with word "odor" | Search |
| Perfumer and Flavorist: | Search |
| Google Patents: | Search |
| US Patents: | Search |
| EU Patents: | Search |
| Pubchem Patents: | Search |
| PubMed: | Search |
| NCBI: | Search |
| JECFA Food Flavoring: | 973 p-mentha-1,8-dien-7-al |
| DG SANTE Food Flavourings: | 05.117 p-mentha-1,8-dien-7-al |
| FEMA Number: | 3557 p-mentha-1,8-dien-7-al |
| FDA: | No longer provide for the use of these seven synthetic flavoring substances |
| FDA Mainterm (SATF): | 2111-75-3 ; PERILLALDEHYDE |
| FDA Regulation: | |
| FDA PART 172 -- FOOD ADDITIVES PERMITTED FOR DIRECT ADDITION TO FOOD FOR HUMAN CONSUMPTION Subpart F--Flavoring Agents and Related Substances Sec. 172.515 Synthetic flavoring substances and adjuvants. | |
Physical Properties:
| Appearance: | pale yellow to dark yellow clear oily liquid (est) |
| Assay: | 97.00 to 100.00 % |
| Food Chemicals Codex Listed: | No |
| Specific Gravity: | 0.94000 to 0.95600 @ 25.00 °C. |
| Pounds per Gallon - (est).: | 7.822 to 7.955 |
| Refractive Index: | 1.50400 to 1.51300 @ 20.00 °C. |
| Optical Rotation: | -125.00 to -118.00 |
| Boiling Point: | 238.00 to 240.00 °C. @ 760.00 mm Hg |
| Vapor Pressure: | 0.043000 mmHg @ 25.00 °C. (est) |
| Flash Point: | 200.00 °F. TCC ( 93.33 °C. ) |
| logP (o/w): | 3.053 (est) |
| Soluble in: | |
| alcohol | |
| oils | |
| water, 160.7 mg/L @ 25 °C (est) | |
| Insoluble in: | |
| water | |
Organoleptic Properties:
| Odor Type: herbal | |
| Odor Strength: | high , recommend smelling in a 10.00 % solution or less |
| Substantivity: | 8 hour(s) at 100.00 % |
| fresh green herbal grassy sweet minty cumin | |
| Odor Description: at 10.00 % in dipropylene glycol. | fresh green herbal grassy sweet mint cumin Luebke, William tgsc, (1986) |
| spicy cumin cinnamon pungent clove citrus orange lime | |
| Odor Description: | Spicy, cumin, cinnamon, pungent, clove, citrus, orange and lime Mosciano, Gerard P&F 22, No. 5, 67, (1997) |
| Flavor Type: spicy | |
| woody spicy waxy sweet citrus lime aldehydic | |
| Taste Description: at 25.00 ppm. | Woody, spicy, waxy, sweet, citrus, lime and aldehydic Mosciano, Gerard P&F 22, No. 5, 67, (1997) |
| Odor and/or flavor descriptions from others (if found). | |
Cosmetic Information:
| CosIng: | cosmetic data |
| Cosmetic Uses: |
fragrance perfuming agents skin conditioning |
Suppliers:
Safety Information:
| European information : | |
| Most important hazard(s): | |
| Xi - Irritant | |
|
R 36/37/38 - Irritating to eyes, respiratory system, and skin. S 02 - Keep out of the reach of children. S 24/25 - Avoid contact with skin and eyes. S 26 - In case of contact with eyes, rinse immediately with plenty of water and seek medical advice. S 37/39 - Wear suitable gloves and eye/face protection. | |
| Hazards identification | |
| Classification of the substance or mixture | |
| GHS Classification in accordance with 29 CFR 1910 (OSHA HCS) | |
| None found. | |
| GHS Label elements, including precautionary statements | |
| Pictogram | |
| Hazard statement(s) | |
| None found. | |
| Precautionary statement(s) | |
| None found. | |
| Oral/Parenteral Toxicity: | |
|
oral-mouse LD50 1720 mg/kg Food and Chemical Toxicology. Vol. 20, Pg. 799, 1982. | |
| Dermal Toxicity: | |
|
skin-guinea pig LD50 > 5000 mg/kg Food and Chemical Toxicology. Vol. 20, Pg. 799, 1982. | |
| Inhalation Toxicity: | |
| Not determined | |
Safety in Use Information:
| Category: | cosmetic, flavor and fragrance agents | ||
| RIFM Fragrance Material Safety Assessment: Search | |||
| IFRA Code of Practice Notification of the 49th Amendment to the IFRA Code of Practice | |||
| IFRA Critical Effect: | Dermal sensitization | ||
| IFRA fragrance material specification: | |||
| Should not be used such that the level in finished cosmetic products exceeds 0.1%. Based on test results showing sensitisation (IFRA guidelines). | |||
| IFRA: | View Standard | ||
| View IFRA Standards Library for complete information. | |||
| Please review Amendment 49 IFRA documentation for complete information. | |||
| IFRA RESTRICTION LIMITS IN THE FINISHED PRODUCT (%): | |||
| Category 1: Products applied to the lips | |||
| 0.054 % | |||
| Category 2: Products applied to the axillae | |||
| 0.016 % | |||
| Category 3: Products applied to the face/body using fingertips | |||
| 0.32 % | |||
| Category 4: Products related to fine fragrance | |||
| 0.30 % | |||
| Category 5: Products applied to the face and body using the hands (palms), primarily leave-on | |||
| Category 5A: Body lotion products applied to the body using the hands (palms), primarily leave-on | |||
| 0.076 % | |||
| Category 5B: Face moisturizer products applied to the face using the hands (palms), primarily leave-on | |||
| 0.076 % | |||
| Category 5C: Hand cream products applied to the hands using the hands (palms), primarily leave-on | |||
| 0.076 % | |||
| Category 5D: Baby Creams, baby Oils and baby talc | |||
| 0.076 % | |||
| Category 6: Products with oral and lip exposure | |||
| 0.18 % | |||
| Category 7: Products applied to the hair with some hand contact | |||
| Category 7A: Rinse-off products applied to the hair with some hand contact | |||
| 0.61 % | |||
| Category 7B: Leave-on products applied to the hair with some hand contact | |||
| 0.61 % | |||
| Category 8: Products with significant anogenital exposure | |||
| 0.032 % | |||
| Category 9: Products with body and hand exposure, primarily rinse off | |||
| 0.59 % | |||
| Category 10: Household care products with mostly hand contact | |||
| Category 10A: Household care excluding aerosol products (excluding aerosol/spray products) | |||
| 2.10 % | |||
| Category 10B: Household aerosol/spray products | |||
| 2.10 % | |||
| Category 11: Products with intended skin contact but minimal transfer of fragrance to skin from inert substrate | |||
| Category 11A: Products with intended skin contact but minimal transfer of fragrance to skin from inert substrate without UV exposure | |||
| 1.20 % | |||
| Category 11B: Products with intended skin contact but minimal transfer of fragrance to skin from inert substrate with potential UV exposure | |||
| 1.20 % | |||
| Category 12: Products not intended for direct skin contact, minimal or insignificant transfer to skin | |||
| No Restriction | |||
| Notes: | |||
| IFRA FLAVOR REQUIREMENTS: | |||
Due to the possible ingestion of small amounts of fragrance ingredients from their use in products in Categories 1 and 6, materials must not only comply with IFRA Standards but must also be recognized as safe as a flavoring ingredient as defined by the IOFI Code of Practice (www.iofi.org). For more details see chapter 1 of the Guidance for the use of IFRA Standards. | |||
| Maximised Survey-derived Daily Intakes (MSDI-EU): | 2.10 (μg/capita/day) | ||
| Maximised Survey-derived Daily Intakes (MSDI-USA): | 2.00 (μg/capita/day) | ||
| Structure Class: | I | ||
| Use levels for FEMA GRAS flavoring substances on which the FEMA Expert Panel based its judgments that the substances are generally recognized as safe (GRAS). | |||
| The Expert Panel also publishes separate extensive reviews of scientific information on all FEMA GRAS flavoring substances and can be found at FEMA Flavor Ingredient Library | |||
| publication number: 11 | |||
| Click here to view publication 11 | |||
| average usual ppm | average maximum ppm | ||
| baked goods: | - | 4.50000 | |
| beverages(nonalcoholic): | - | 4.00000 | |
| beverages(alcoholic): | - | 6.00000 | |
| breakfast cereal: | - | - | |
| cheese: | - | - | |
| chewing gum: | - | - | |
| condiments / relishes: | - | - | |
| confectionery froastings: | - | - | |
| egg products: | - | - | |
| fats / oils: | - | - | |
| fish products: | - | - | |
| frozen dairy: | - | 6.30000 | |
| fruit ices: | - | - | |
| gelatins / puddings: | - | 4.50000 | |
| granulated sugar: | - | - | |
| gravies: | - | - | |
| hard candy: | - | - | |
| imitation dairy: | - | - | |
| instant coffee / tea: | - | - | |
| jams / jellies: | - | - | |
| meat products: | - | 20.00000 | |
| milk products: | - | - | |
| nut products: | - | - | |
| other grains: | - | - | |
| poultry: | - | - | |
| processed fruits: | - | - | |
| processed vegetables: | - | - | |
| reconstituted vegetables: | - | - | |
| seasonings / flavors: | - | - | |
| snack foods: | - | - | |
| soft candy: | - | 9.70000 | |
| soups: | - | - | |
| sugar substitutes: | - | - | |
| sweet sauces: | - | - | |
Safety References:
| Flavor & Extract Manufacturers Association (FEMA) reference(s): | |
| The FEMA GRAS assessment of alicyclic substances used as flavor ingredients. View pdf | |
| European Food Safety Athority(EFSA): | Flavor usage levels; Subacute, Subchronic, Chronic and Carcinogenicity Studies; Developmental / Reproductive Toxicity Studies; Genotoxicity Studies... |
| European Food Safety Authority (EFSA) reference(s): | |
| List of apha, beta-Unsaturated Aldehydes and Ketones representative of FGE.19 substances for Genotoxicity Testing [1] - Statement of the Panel on Food Contact Materials, Enzymes, Flavourings and Processing Aids (CEF) View page or View pdf | |
| Scientific Opinion on Flavouring Group Evaluation 208 Revision 1 (FGE.208Rev1): Consideration of genotoxicity data on representatives for 10 alicyclic aldehydes with the a,ß-unsaturation in ring / side-chain and precursors from chemical subgroup 2.2 of View page or View pdf | |
| Scientific Opinion on Flavouring Group Evaluation 208 Revision 2 (FGE.208Rev2): Consideration of genotoxicity data on alicyclic aldehydes with a,ß-unsaturation in ring/side-chain and precursors from chemical subgroup 2.2 of FGE.19 View page or View pdf | |
| Scientific Opinion on Flavouring Group Evaluation 208 Revision 2 (FGE.208Rev2): Consideration of genotoxicity data on alicyclic aldehydes with a,ß-unsaturation in ring/side-chain and precursors from chemical subgroup 2.2 of FGE.19 View page or View pdf | |
| Scientific Opinion on Flavouring Group Evaluation 208 Revision 3 (FGE.208Rev3): consideration of genotoxicity data on alicyclic aldehydes with a,ß-unsaturation in ring/side-chain and precursors from chemical subgroup 2.2 of FGE.19 View page or View pdf | |
| EPI System: | View |
| Chemical Carcinogenesis Research Information System: | Search |
| AIDS Citations: | Search |
| Cancer Citations: | Search |
| Toxicology Citations: | Search |
| EPA Substance Registry Services (TSCA): | 2111-75-3 |
| EPA ACToR: | Toxicology Data |
| EPA Substance Registry Services (SRS): | Registry |
| Laboratory Chemical Safety Summary : | 16441 |
| National Institute of Allergy and Infectious Diseases: | Data |
| SCCNFP: | opinion |
| WGK Germany: | 3 |
| 4-prop-1-en-2-ylcyclohexene-1-carbaldehyde | |
| Chemidplus: | 0002111753 |
References:
| 4-prop-1-en-2-ylcyclohexene-1-carbaldehyde | |
| NIST Chemistry WebBook: | Search Inchi |
| Canada Domestic Sub. List: | 2111-75-3 |
| Pubchem (cid): | 16441 |
| Pubchem (sid): | 134982357 |
| Flavornet: | 2111-75-3 |
| Pherobase: | View |
Other Information:
| (IUPAC): | Atomic Weights of the Elements 2011 (pdf) |
| Videos: | The Periodic Table of Videos |
| tgsc: | Atomic Weights use for this web site |
| (IUPAC): | Periodic Table of the Elements |
| FDA Substances Added to Food (formerly EAFUS): | View |
| CHEBI: | View |
| CHEMBL: | View |
| Golm Metabolome Database: | Search |
| UM BBD: | Search |
| KEGG (GenomeNet): | C02576 |
| HMDB (The Human Metabolome Database): | HMDB03647 |
| FooDB: | FDB014789 |
| Export Tariff Code: | 2912.49.0000 |
| Typical G.C. | |
| VCF-Online: | VCF Volatile Compounds in Food |
| ChemSpider: | View |
| Wikipedia: | View |
Potential Blenders and core components note
Potential Uses:
| apple | FR | |
| blackberry | FR | |
| citrus | FR | |
| cumin oil replacer | FR | |
| grapefruit | FR | |
| grass | ||
| green | FR | |
| herbal | FR | |
| labdanum | FR | |
| lime | FR | |
| mandarin | FR | |
| mint | FR | |
| orange | FR | |
| peppermint | FR | |
| spearmint | FR | |
| tea black tea | FL | |
| tea green tea | FR | |
| woody | FR |
Occurrence (nature, food, other): note
| amomum testaceum ridl. fruit oil malaysia @ 0.30% Data GC Search Trop Picture | |
| blackberry fruit Search PMC Picture | |
| blood orange oil (citrus sinensis l. var. sanguinello) italy @ 0.009% Data GC Search Trop Picture | |
| blood orange oil (citrus sinensis l. var. tarocco) italy @ 0.008% Data GC Search Trop Picture | |
| blood orange oil italy @ 0.010% Data GC Search Trop Picture | |
| caraway seed Search Trop Picture | |
| celery seed Search Trop Picture | |
| costmary oil @ 0.43% Data GC Search Trop Picture | |
| cumin seed Search Trop Picture | |
| cumin seed oil turkey @ 2.4% Data GC Search Trop Picture | |
| fleabane oil @ 0.10% Data GC Search Trop Picture | |
| ginger rhizome oil Search Trop Picture | |
| gingergrass oil @ 0.21% Data GC Search Trop Picture | |
| grapefruit juice Search Trop Picture | |
| grapefruit oil Search Trop Picture | |
| grapefruit oil c.p. @ 0.03% Data GC Search Trop Picture | |
| grapefruit oil california @ 0.05% Data GC Search Trop Picture | |
| grapefruit oil CO2 extract @ 0.05% Data GC Search Trop Picture | |
| labdanum siam Search PMC Picture | |
| lemon fruit Search Trop Picture | |
| lemon oil california @ 0.03% Data GC Search Trop Picture | |
| lime fruit Search Trop Picture | |
| lime fruit juice Search Trop Picture | |
| lime oil distilled mexico @ 0.02% Data GC Search Trop Picture | |
| lime oil distilled peru @ 0.05% Data GC Search Trop Picture | |
| lime oil expressed florida @ trace% Data GC Search Trop Picture | |
| lime peel oil Search Trop Picture | |
| mandarin fruit Search Trop Picture | |
| mandarin oil @ 0.53% Data GC Search Trop Picture | |
| mandarin oil @ trace% Data GC Search Trop Picture | |
| mandarin oil italy @ trace% Data GC Search Trop Picture | |
| mandarin oil uruguay @ 0.02-0.04% Data GC Search Trop Picture | |
| mandarin peel Search Trop Picture | |
| mikan peel oil @ trace% Data GC Search Trop Picture | |
| orange fruit Search Trop Picture | |
| orange oil Search Trop Picture | |
| orange oil terpeneless @ 0.80% Data GC Search Trop Picture | |
| orange peel oil bitter china @ 0.01% Data GC Search Trop Picture | |
| orange peel oil bitter italy @ 0.03% Data GC Search Trop Picture | |
| orange peel oil sweet c.p. blond italy @ 0.009% Data GC Search Trop Picture | |
| orange peel oil sweet c.p. blond italy @ 0.013% Data GC Search Trop Picture | |
| orange peel oil sweet c.p. blond italy @ 0.014% Data GC Search Trop Picture | |
| orange peel oil sweet c.p. blond italy @ 0.015% Data GC Search Trop Picture | |
| orange peel oil sweet c.p. blond italy @ 0.015% Data GC Search Trop Picture | |
| orange peel oil sweet c.p. florida @ 0.01% Data GC Search Trop Picture | |
| orange peel oil sweet c.p. valencia @ 0.01% Data GC Search Trop Picture | |
| pepper black pepper fruit Search Trop Picture | |
| peppermint Search Trop Picture | |
| spearmint oil Search Trop Picture | |
| tea black tea Search Trop Picture | |
| tsauri grass oil @ 0.90% Data GC Search Trop Picture | |
| yuzu peel oil c.p. @ 0.01-0.02% Data GC Search Trop Picture | |
| yuzu peel oil CO2 extract @ trace-0.05% Data GC Search Trop Picture |
Synonyms:
| 1- | cyclohexene-1-carboxaldehyde, 4-(1-methylethenyl)- |
| 1- | cyclohexene-1-carboxaldehyde, 4-isopropenyl- |
| dihydro cuminyl aldehyde | |
| dihydro cuminyl aldehyde natural | |
| dihydrocuminic aldehyde | |
| dihydrocuminyl aldehyde | |
| dihydrocuminylaldehyde | |
| p- | mentha-1,8-dien-7-al |
| para- | mentha-1,8-dien-7-al |
| 1,8-p- | menthadien-7-al |
| 4-(1- | methyl ethenyl)-1-cyclohexene-1-carboxaldehyde |
| 4-(1- | methylethenyl)-1-cyclohexene-1-carboxaldehyde |
| 4-(1- | methylethenyl)-1-cyclohexene1-carboxyaldehyde |
| perilla aldehyde | |
| perilla aldehyde natural | |
| perillaaldehyde | |
| perillal | |
| DL- | perillaldehyde |
| perillic aldehyde | |
| perillyl aldehyde | |
| perillylaldehyde | |
| 4-( | prop-1-en-2-yl)cyclohex-1-ene-1-carbaldehyde |
| 4- | prop-1-en-2-ylcyclohexene-1-carbaldehyde |
| 4-iso | propenyl cyclohex-1-ene carbaldehyde |
| 4-iso | propenyl-1-cyclohexene-1-carbaldehyde |
| 4-iso | propenyl-1-cyclohexene-1-carboxaldehyde |
| 4-iso | propenylcyclohex-1-enecarbaldehyde |
















