Articles:
2-butyl alcohol
Notes:
Isol. from fruit of blackcurrant (Ribes nigrum) and other fruits. Also present in various cheeses, wines, black tea, endive and clary sage. Flavouring agent
2-Butanol, or sec-butanol, is an organic compound with formula CH3CH(OH)CH2CH3. This secondary alcohol is a flammable, colorless liquid that is soluble in 12 parts water and completely miscible with polar organic solvents such as ethers and other alcohols. It is produced on a large scale, primarily as a precursor to the industrial solvent methyl ethyl ketone. 2-Butanol is chiral and thus can be obtained as either of two stereoisomers designated as (R)-(-)-2-butanol and (S)-(+)-2-butanol. It is normally found as an equal mixture of the two stereoisomers — a racemic mixture. (Wikipedia)
| CAS Number: | 78-92-2 | 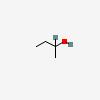 3D/inchi 3D/inchi
|
| Other(deleted CASRN): | 15892-23-6 | |
| ECHA EINECS - REACH Pre-Reg: | 201-158-5 | |
| FDA UNII: | 0TUL3ENK62 | |
| Nikkaji Web: | J2.832K | |
| Beilstein Number: | 0773649 | |
| MDL: | MFCD00004569 | |
| CoE Number: | 11735 | |
| XlogP3: | 0.60 (est) | |
| Molecular Weight: | 74.12290000 | |
| Formula: | C4 H10 O | |
| BioActivity Summary: | listing | |
| NMR Predictor: | Predict (works with chrome or firefox) | |
Category: flavor and fragrance agents, extraction solvent
US / EU / FDA / JECFA / FEMA / FLAVIS / Scholar / Patent Information:
| Google Scholar: | Search |
| Google Books: | Search |
| Google Scholar: with word "volatile" | Search |
| Google Scholar: with word "flavor" | Search |
| Google Scholar: with word "odor" | Search |
| Perfumer and Flavorist: | Search |
| Google Patents: | Search |
| US Patents: | Search |
| EU Patents: | Search |
| Pubchem Patents: | Search |
| PubMed: | Search |
| NCBI: | Search |
| JECFA Food Additive: | Butan-2-ol |
| DG SANTE Food Flavourings: | 02.121 butan-2-ol |
| FDA Mainterm (SATF): | 78-92-2 ; 2-BUTANOL |
| FDA Regulation: | |
| FDA PART 172 -- FOOD ADDITIVES PERMITTED FOR DIRECT ADDITION TO FOOD FOR HUMAN CONSUMPTION Subpart F--Flavoring Agents and Related Substances Sec. 172.515 Synthetic flavoring substances and adjuvants. FDA PART 176 -- INDIRECT FOOD ADDITIVES: PAPER AND PAPERBOARD COMPONENTS Subpart B--Substances for Use Only as Components of Paper and Paperboard Sec. 176.180 Components of paper and paperboard in contact with dry food. | |
Physical Properties:
| Appearance: | colorless clear liquid (est) |
| Assay: | 98.00 to 100.00 % |
| Food Chemicals Codex Listed: | No |
| Specific Gravity: | 0.80600 to 0.80800 @ 20.00 °C. |
| Pounds per Gallon - (est).: | 6.715 to 6.731 |
| Refractive Index: | 1.39600 to 1.39800 @ 25.00 °C. |
| Melting Point: | -115.00 °C. @ 760.00 mm Hg |
| Boiling Point: | 99.50 °C. @ 760.00 mm Hg |
| Vapor Pressure: | 18.300000 mmHg @ 20.00 °C. |
| Vapor Density: | 2.6 ( Air = 1 ) |
| Flash Point: | 80.00 °F. TCC ( 26.67 °C. ) |
| logP (o/w): | 0.610 |
| Soluble in: | |
| alcohol | |
| water, 1.81E+05 mg/L @ 25 °C (exp) | |
Organoleptic Properties:
| Odor Type: fruity | |
| sweet apricot | |
| Odor Description: at 10.00 % in dipropylene glycol. | sweet apricot |
| Odor and/or flavor descriptions from others (if found). | |
Cosmetic Information:
| CosIng: | cosmetic data |
| Cosmetic Uses: |
perfuming agents |
Suppliers:
| CJ Latta & Associates |
| 2-BUTANOL |
| ECSA Chemicals |
| BUTAN-2-OL RPE-FOR ANALYSIS-REAG. PH.EUR., M |
| ECSA TRADE THE MOST UPDATED FINANCIAL PUBLICATION ON THE WORLD OF CHEMISTRY |
| EMD Millipore |
| For experimental / research use only. |
| 2-Butanol |
| Glentham Life Sciences |
| 2-Butanol |
| Penta International |
| 2-BUTANOL |
| Santa Cruz Biotechnology |
| For experimental / research use only. |
| 2-Butanol |
| Sigma-Aldrich |
| For experimental / research use only. |
| 2-Butanol analytical standard |
| TCI AMERICA |
| For experimental / research use only. |
| 2-Butanol >99.0%(GC) |
Safety Information:
| Preferred SDS: View | |
| European information : | |
| Most important hazard(s): | |
| Xn - Harmful. | |
|
R 10 - Flammable. R 36/37 - Irritating to eyes and respiratory system. R 67 - Vapours may cause frowsiness and dizziness. S 02 - Keep out of the reach of children. S 07/09 - Keep container tightly closed and in well-ventilated place. S 13 - Keep away from food, drink and animal feedingstuffs. S 24/25 - Avoid contact with skin and eyes. S 26 - In case of contact with eyes, rinse immediately with plenty of water and seek medical advice. S 46 - If swallowed, seek medical advice immediately and show this container or label. | |
| Hazards identification | |
| Classification of the substance or mixture | |
| GHS Classification in accordance with 29 CFR 1910 (OSHA HCS) | |
| None found. | |
| GHS Label elements, including precautionary statements | |
| Pictogram | |
| Hazard statement(s) | |
| None found. | |
| Precautionary statement(s) | |
| None found. | |
| Oral/Parenteral Toxicity: | |
|
oral-rat LD50 2193 mg/kg BEHAVIORAL: COMA BEHAVIORAL: SOMNOLENCE (GENERAL DEPRESSED ACTIVITY) BEHAVIORAL: ATAXIA National Technical Information Service. Vol. OTS0557575 intravenous-rat LD50 138 mg/kg EHP, Environmental Health Perspectives. Vol. 61, Pg. 321, 1985. intraperitoneal-rat LD50 1193 mg/kg EHP, Environmental Health Perspectives. Vol. 61, Pg. 321, 1985. oral-rabbit LD50 4893 mg/kg LUNGS, THORAX, OR RESPIRATION: DYSPNEA CARDIAC: PULSE RATE SENSE ORGANS AND SPECIAL SENSES: CORNEAL DAMAGE: EYE Industrial Medicine and Surgery. Vol. 41, Pg. 31, 1972. intraperitoneal-rabbit LD50 277 mg/kg EHP, Environmental Health Perspectives. Vol. 61, Pg. 321, 1985. intravenous-mouse LD50 764 mg/kg Archives Internationales de Pharmacodynamie et de Therapie. Vol. 135, Pg. 330, 1962. intraperitoneal-mouse LD50 771 mg/kg Shell Chemical Company. Unpublished Report. Vol. -, Pg. 2, 1961. intraperitoneal-hamster LD50 1218 mg/kg EHP, Environmental Health Perspectives. Vol. 61, Pg. 321, 1985. intraperitoneal-guinea pig LD50 1067 mg/kg EHP, Environmental Health Perspectives. Vol. 61, Pg. 321, 1985. oral-dog LDLo 2400 mg/kg National Technical Information Service. Vol. OTS0572377 parenteral-frog LDLo 15000 mg/kg BEHAVIORAL: SOMNOLENCE (GENERAL DEPRESSED ACTIVITY) PERIPHERAL NERVE AND SENSATION: SPASTIC PARALYSIS WITH OR WITHOUT SENSORY CHANGE Archives Internationales de Pharmacodynamie et de Therapie. Vol. 50, Pg. 296, 1935. | |
| Dermal Toxicity: | |
|
skin-rat LD50 > 2000 mg/kg National Technical Information Service. Vol. OTS0557575 | |
| Inhalation Toxicity: | |
|
inhalation-rat LCLo 16000 ppm/4H SENSE ORGANS AND SPECIAL SENSES: OTHER CHANGES: OLFACTION BEHAVIORAL: SOMNOLENCE (GENERAL DEPRESSED ACTIVITY) LUNGS, THORAX, OR RESPIRATION: OTHER CHANGES Shell Chemical Company. Vol. MSDS-5280-4 | |
Safety in Use Information:
| Category: | flavor and fragrance agents, extraction solvent | ||
| RIFM Fragrance Material Safety Assessment: Search | |||
| IFRA Code of Practice Notification of the 49th Amendment to the IFRA Code of Practice | |||
Safety References:
| European Food Safety Authority (EFSA) reference(s): | |
| Flavouring Group Evaluation 7, Revision 1 (FGE.07Rev1): Saturated and unsaturated aliphatic secondary alcohols, ketones and esters of secondary alcohols and saturated linear or branched-chain carboxylic acids from chemical group 5 (Commission Regulation (EC) No 1565/2000 of 18 July 2000) - Scientific opinion of the Panel on Food Additives, Flavourings, Processing Aids and Materials in contact with Food (AFC) on a request from the Commission View page or View pdf | |
| Flavouring Group Evaluation 10, Revision 1 (FGE10 Rev1)[1] - Aliphatic primary and secondary saturated and unsaturated alcohols, aldehydes, acetals, carboxylic acids and esters containing an additional oxygenated functional group and lactones from chemical groups 9, 13 and 30 - Scientific Opinion of the Panel on Food Additives, Flavourings, Processing Aids and Materials in Contact with Food (AFC) View page or View pdf | |
| Flavouring Group Evaluation 7, Revision 2 (FGE.07Rev2) : Saturated and unsaturated aliphatic secondary alcohols, ketones and esters of secondary alcohols and saturated linear or branched-chain carboxylic acids from chemical group 5 View page or View pdf | |
| Risk Assessment of Food Contact Materials View page or View pdf | |
| EPI System: | View |
| NIOSH International Chemical Safety Cards: | search |
| NIOSH Pocket Guide: | search |
| AIDS Citations: | Search |
| Cancer Citations: | Search |
| Toxicology Citations: | Search |
| EPA Substance Registry Services (TSCA): | 78-92-2 |
| EPA ACToR: | Toxicology Data |
| EPA Substance Registry Services (SRS): | Registry |
| Laboratory Chemical Safety Summary : | 6568 |
| National Institute of Allergy and Infectious Diseases: | Data |
| WISER: | UN 1120 |
| WGK Germany: | 1 |
| butan-2-ol | |
| Chemidplus: | 0000078922 |
| EPA/NOAA CAMEO: | hazardous materials |
| RTECS: | 78-92-2 |
References:
| butan-2-ol | |
| NIST Chemistry WebBook: | Search Inchi |
| Canada Domestic Sub. List: | 78-92-2 |
| Pubchem (cid): | 6568 |
| Pubchem (sid): | 134971087 |
| Flavornet: | 78-92-2 |
| Pherobase: | View |
Other Information:
| (IUPAC): | Atomic Weights of the Elements 2011 (pdf) |
| Videos: | The Periodic Table of Videos |
| tgsc: | Atomic Weights use for this web site |
| (IUPAC): | Periodic Table of the Elements |
| FDA Substances Added to Food (formerly EAFUS): | View |
| CHEBI: | View |
| CHEMBL: | View |
| Golm Metabolome Database: | Search |
| HMDB (The Human Metabolome Database): | HMDB11469 |
| FooDB: | FDB003382 |
| Export Tariff Code: | 2905.14.5050 |
| Typical G.C. | |
| VCF-Online: | VCF Volatile Compounds in Food |
| ChemSpider: | View |
| Wikipedia: | View |
| Formulations/Preparations: grades: technical | |
Potential Blenders and core components note
Potential Uses:
| apple | FR | |
| apricot | FR | |
| orange | FR | |
| peach | FR |
Occurrence (nature, food, other): note
| apple fruit juice 677 ppm Search Trop Picture | |
| apple plant Search Trop Picture | |
| bilberry fruit juice Search Trop Picture | |
| blueberry fruit juice Search Trop Picture | |
| butter 115 ppm Search PMC Picture | |
| cheese cheddar cheese 16191 ppm Search PMC Picture | |
| currant black currant fruit Search Trop Picture | |
| ginger oil Search Trop Picture | |
| mikan peel oil @ 0.01% Data GC Search Trop Picture | |
| orange juice 74 ppm Search Trop Picture | |
| potato chip 9 ppm Search PMC Picture | |
| rosemary sap Search Trop Picture | |
| tea leaf Search Trop Picture |
Synonyms:
| butan-2-ol | |
| DL- | butan-2-ol |
| (±)-2- | butanol |
| ±-2- | butanol |
| DL-sec- | butanol |
| dextro,laevo-sec- | butanol |
| rac-2- | butanol |
| racemic-2- | butanol |
| s- | butanol |
| sec- | butanol |
| (±)-sec- | butyl alcohol |
| 2- | butyl alcohol |
| s- | butyl alcohol |
| sec- | butyl alcohol |
| butyl alcohol, sec- | |
| 2- | butylalcohol |
| butylene hydrate | |
| ethyl methyl carbinol | |
| ethylmethyl carbinol | |
| ethylmethylcarbinol | |
| 2- | hydroxybutane |
| methyl ethyl carbinol | |
| dextro,laevo- | methyl ethyl carbinol |
| 1- | methyl propanol |
| 1- | methyl propyl alcohol |
| 1- | methyl-1-propanol |
| methylethyl carbinol | |
| DL- | methylethyl carbinol |
| methylethylcarbinol | |
| 1- | methylpropanol |
| 1- | methylpropyl alcohol |




