Articles:
2-formyl-6,6-dimethylbicyclo[3.1.1]hept-2-ene (myrtenal)
Notes:
Occurs in orange, lemon, spearmint, pepper, thyme, juniper, calamus, ginger, myrtle, lemon balm, calabash, nutmeg, parsley seed and other plant oils
| CAS Number: | 564-94-3 | 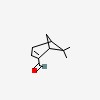 3D/inchi 3D/inchi
|
| Other(deleted CASRN): | 57526-63-3 | |
| ECHA EINECS - REACH Pre-Reg: | 209-274-8 | |
| FDA UNII: | 8J97443QRZ | |
| Nikkaji Web: | J121.133A | |
| Beilstein Number: | 2961587 | |
| MDL: | MFCD00074768 | |
| CoE Number: | 10379 | |
| XlogP3-AA: | 2.10 (est) | |
| Molecular Weight: | 150.22078000 | |
| Formula: | C10 H14 O | |
| NMR Predictor: | Predict (works with chrome, Edge or firefox) | |
| EFSA/JECFA Comments: | According to the information provided, the substance is a racemate (Documentation provided to EFSA n. 3) | |
Category: flavor and fragrance agents
US / EU / FDA / JECFA / FEMA / FLAVIS / Scholar / Patent Information:
| Google Scholar: | Search |
| Google Books: | Search |
| Google Scholar: with word "volatile" | Search |
| Google Scholar: with word "flavor" | Search |
| Google Scholar: with word "odor" | Search |
| Perfumer and Flavorist: | Search |
| Google Patents: | Search |
| US Patents: | Search |
| EU Patents: | Search |
| Pubchem Patents: | Search |
| PubMed: | Search |
| NCBI: | Search |
| JECFA Food Flavoring: | 980 2-formyl-6,6-dimethylbicyclo[3.1.1]hept-2-ene (myrtenal) |
| DG SANTE Food Flavourings: | 05.106 myrtenal |
| FEMA Number: | 3395 2-formyl-6,6-dimethylbicyclo[3.1.1]hept-2-ene (myrtenal) |
| FDA: | No longer provide for the use of these seven synthetic flavoring substances |
| FDA Mainterm (SATF): | 564-94-3 ; 2-FORMYL-6,6-DIMETHYLBICYCLO(3.1.1)HEPT-2-ENE |
Physical Properties:
| Appearance: | colorless clear liquid (est) |
| Assay: | 98.00 to 100.00 % |
| Food Chemicals Codex Listed: | No |
| Specific Gravity: | 0.98400 to 0.99000 @ 25.00 °C. |
| Pounds per Gallon - (est).: | 8.188 to 8.238 |
| Refractive Index: | 1.49600 to 1.50700 @ 20.00 °C. |
| Boiling Point: | 220.00 to 221.00 °C. @ 760.00 mm Hg |
| Boiling Point: | 99.00 to 100.00 °C. @ 15.00 mm Hg |
| Vapor Pressure: | 0.145000 mmHg @ 25.00 °C. (est) |
| Flash Point: | 174.00 °F. TCC ( 78.89 °C. ) |
| logP (o/w): | 2.980 |
| Soluble in: | |
| alcohol | |
| dipropylene glycol | |
| water, 215.9 mg/L @ 25 °C (est) | |
| Insoluble in: | |
| water | |
Organoleptic Properties:
| Odor Type: spicy | |
| Odor Strength: | medium |
| Substantivity: | 48 hour(s) at 100.00 % |
| sweet spicy cinnamon tonka terpenic camphoreous jammy | |
| Odor Description: at 100.00 %. | sweet cinnamon tonka spicy terpene camphor jam Luebke, William tgsc, (1996) |
| cooling green minty spicy woody | |
| Odor Description: | Cooling, green, minty with spicy woody notes Mosciano, Gerard P&F 15, No. 1, 19, (1990) |
| Flavor Type: minty | |
| minty green cooling powdery spicy | |
| Taste Description: at 30.00 ppm. | Minty, green and cooling with powdery spicy notes Mosciano, Gerard P&F 15, No. 1, 19, (1990) |
| Odor and/or flavor descriptions from others (if found). | |
Cosmetic Information:
| CosIng: | cosmetic data |
| Cosmetic Uses: |
perfuming agents |
Suppliers:
| BOC Sciences |
| For experimental / research use only. |
| Bicyclo[3.1.1]hept-2-ene-2-carboxaldehyde,6,6-dimethyl- |
| Ernesto Ventós |
| MYRTENAL
Odor: REFRESHING, HERBACEOUS, SPICY |
| Frutarom |
| MYRTENAL
KOSHER Flavor: Sweet, Spicy, Terpenic, Green, Cooling |
| IFF |
| MYRTENAL
KOSHER Flavor: Sweet, Spicy, Terpenic, Green, Cooling |
| Lluch Essence |
| MYRTENAL |
| Moellhausen |
| MYRTENAL |
| Nippon Terpene Chemicals |
| Myrtenal 93% up |
| Penta International |
| MYRTENAL |
| Synerzine |
| Myrtenal |
Safety Information:
| European information : | |
| Most important hazard(s): | |
| None - None found. | |
|
S 02 - Keep out of the reach of children. S 24/25 - Avoid contact with skin and eyes. | |
| Hazards identification | |
| Classification of the substance or mixture | |
| GHS Classification in accordance with 29 CFR 1910 (OSHA HCS) | |
| None found. | |
| GHS Label elements, including precautionary statements | |
| Pictogram | |
| Hazard statement(s) | |
| None found. | |
| Precautionary statement(s) | |
| None found. | |
| Oral/Parenteral Toxicity: | |
|
oral-rat LD50 2300 mg/kg Food and Chemical Toxicology. Vol. 26, Pg. 329, 1988. intravenous-mouse LD50 170 mg/kg Food and Chemical Toxicology. Vol. 26, Pg. 329, 1988. | |
| Dermal Toxicity: | |
|
skin-rabbit LD50 > 5000 mg/kg Food and Chemical Toxicology. Vol. 26, Pg. 329, 1988. | |
| Inhalation Toxicity: | |
| Not determined | |
Safety in Use Information:
| Category: | flavor and fragrance agents | ||
| RIFM Fragrance Material Safety Assessment: Search | |||
| IFRA Code of Practice Notification of the 49th Amendment to the IFRA Code of Practice | |||
| Recommendation for myrtenal usage levels up to: | |||
| 2.0000 % in the fragrance concentrate. | |||
| Maximised Survey-derived Daily Intakes (MSDI-EU): | 2.20 (μg/capita/day) | ||
| Maximised Survey-derived Daily Intakes (MSDI-USA): | 7.00 (μg/capita/day) | ||
| Modified Theoretical Added Maximum Daily Intake (mTAMDI): | 1700 (μg/person/day) | ||
| Threshold of Concern: | 1800 (μg/person/day) | ||
| Structure Class: | I | ||
| Use levels for FEMA GRAS flavoring substances on which the FEMA Expert Panel based its judgments that the substances are generally recognized as safe (GRAS). | |||
| The Expert Panel also publishes separate extensive reviews of scientific information on all FEMA GRAS flavoring substances and can be found at FEMA Flavor Ingredient Library | |||
| publication number: 7 | |||
| Click here to view publication 7 | |||
| average usual ppm | average maximum ppm | ||
| baked goods: | - | - | |
| beverages(nonalcoholic): | - | 0.50000 | |
| beverages(alcoholic): | - | - | |
| breakfast cereal: | - | - | |
| cheese: | - | - | |
| chewing gum: | - | - | |
| condiments / relishes: | - | - | |
| confectionery froastings: | - | - | |
| egg products: | - | - | |
| fats / oils: | - | - | |
| fish products: | - | - | |
| frozen dairy: | - | - | |
| fruit ices: | - | - | |
| gelatins / puddings: | - | - | |
| granulated sugar: | - | - | |
| gravies: | - | - | |
| hard candy: | - | 2.00000 | |
| imitation dairy: | - | - | |
| instant coffee / tea: | - | - | |
| jams / jellies: | - | - | |
| meat products: | - | - | |
| milk products: | - | - | |
| nut products: | - | - | |
| other grains: | - | - | |
| poultry: | - | - | |
| processed fruits: | - | - | |
| processed vegetables: | - | - | |
| reconstituted vegetables: | - | - | |
| seasonings / flavors: | - | - | |
| snack foods: | - | - | |
| soft candy: | - | - | |
| soups: | - | - | |
| sugar substitutes: | - | - | |
| sweet sauces: | - | - | |
| Food categories according to Commission Regulation EC No. 1565/2000 (EC, 2000) in FGE.06 (EFSA, 2002a). According to the Industry the "normal" use is defined as the average of reported usages and "maximum use" is defined as the 95th percentile of reported usages (EFSA, 2002i). | |||
| Note: mg/kg = 0.001/1000 = 0.000001 = 1/1000000 = ppm. | |||
| average usage mg/kg | maximum usage mg/kg | ||
| Dairy products, excluding products of category 02.0 (01.0): | 1.00000 | 5.00000 | |
| Fats and oils, and fat emulsions (type water-in-oil) (02.0): | 8.00000 | 50.00000 | |
| Edible ices, including sherbet and sorbet (03.0): | 2.00000 | 10.00000 | |
| Processed fruit (04.1): | 1.00000 | 5.00000 | |
| Processed vegetables (incl. mushrooms & fungi, roots & tubers, pulses and legumes), and nuts & seeds (04.2): | 5.00000 | 20.00000 | |
| Confectionery (05.0): | 20.00000 | 100.00000 | |
| Chewing gum (05.0): | - | - | |
| Cereals and cereal products, incl. flours & starches from roots & tubers, pulses & legumes, excluding bakery (06.0): | 1.00000 | 5.00000 | |
| Bakery wares (07.0): | 5.00000 | 20.00000 | |
| Meat and meat products, including poultry and game (08.0): | 1.00000 | 5.00000 | |
| Fish and fish products, including molluscs, crustaceans and echinoderms (MCE) (09.0): | 1.00000 | 5.00000 | |
| Eggs and egg products (10.0): | 1.00000 | 5.00000 | |
| Sweeteners, including honey (11.0): | 1.00000 | 5.00000 | |
| Salts, spices, soups, sauces, salads, protein products, etc. (12.0): | 5.00000 | 20.00000 | |
| Foodstuffs intended for particular nutritional uses (13.0): | - | - | |
| Non-alcoholic ("soft") beverages, excl. dairy products (14.1): | 1.00000 | 5.00000 | |
| Alcoholic beverages, incl. alcohol-free and low-alcoholic counterparts (14.2): | 1.00000 | 5.00000 | |
| Ready-to-eat savouries (15.0): | 1.00000 | 5.00000 | |
| Composite foods (e.g. casseroles, meat pies, mincemeat) - foods that could not be placed in categories 01.0 - 15.0 (16.0): | 1.00000 | 5.00000 | |
Safety References:
| Flavor & Extract Manufacturers Association (FEMA) reference(s): | |
| The FEMA GRAS assessment of alicyclic substances used as flavor ingredients. View pdf | |
| European Food Safety Athority(EFSA): | Flavor usage levels; Subacute, Subchronic, Chronic and Carcinogenicity Studies; Developmental / Reproductive Toxicity Studies; Genotoxicity Studies... |
| European Food Safety Authority (EFSA) reference(s): | |
| Scientific Opinion on Flavouring Group Evaluation 208 Revision 1 (FGE.208Rev1): Consideration of genotoxicity data on representatives for 10 alicyclic aldehydes with the a,ß-unsaturation in ring / side-chain and precursors from chemical subgroup 2.2 of View page or View pdf | |
| Scientific Opinion on Flavouring Group Evaluation 208 Revision 2 (FGE.208Rev2): Consideration of genotoxicity data on alicyclic aldehydes with a,ß-unsaturation in ring/side-chain and precursors from chemical subgroup 2.2 of FGE.19 View page or View pdf | |
| Scientific Opinion on Flavouring Group Evaluation 73,
Revision 4 (FGE.73Rev4): consideration of alicyclic alcohols,
aldehydes, acids and related esters evaluated by JECFA
(59th and 63rd meeting) structurally related to primary
saturated or unsaturated alicyclic alcohols, aldehydes,
acids and esters evaluated by EFSA in FGE.12Rev5 View page or View pdf | |
| Scientific Opinion on Flavouring Group Evaluation 208 Revision 3 (FGE.208Rev3): consideration of genotoxicity data on alicyclic aldehydes with a,ß-unsaturation in ring/side-chain and precursors from chemical subgroup 2.2 of FGE.19 View page or View pdf | |
| Scientific Opinion on Flavouring Group Evaluation 73, Revision 5 (FGE.73Rev5): consideration of alicyclic alcohols, aldehydes, acids and related esters evaluated by JECFA (59th, 63rd and 86th meeting) and structurally related to substances evaluated in FGE.12Rev5 View page or View pdf | |
| EPI System: | View |
| AIDS Citations: | Search |
| Cancer Citations: | Search |
| Toxicology Citations: | Search |
| EPA Substance Registry Services (TSCA): | 564-94-3 |
| EPA ACToR: | Toxicology Data |
| EPA Substance Registry Services (SRS): | Registry |
| Laboratory Chemical Safety Summary : | 61130 |
| National Institute of Allergy and Infectious Diseases: | Data |
| WGK Germany: | 2 |
| 7,7-dimethylbicyclo[3.1.1]hept-3-ene-4-carbaldehyde | |
| Chemidplus: | 0000564943 |
| RTECS: | DT5180000 for cas# 564-94-3 |
References:
| 7,7-dimethylbicyclo[3.1.1]hept-3-ene-4-carbaldehyde | |
| NIST Chemistry WebBook: | Search Inchi |
| Canada Domestic Sub. List: | 564-94-3 |
| Pubchem (cid): | 61130 |
| Pubchem (sid): | 135017862 |
| Pherobase: | View |
Other Information:
| (IUPAC): | Atomic Weights of the Elements 2011 (pdf) |
| Videos: | The Periodic Table of Videos |
| tgsc: | Atomic Weights use for this web site |
| (IUPAC): | Periodic Table of the Elements |
| FDA Substances Added to Food (formerly EAFUS): | View |
| UM BBD: | Search |
| KEGG (GenomeNet): | C11939 |
| HMDB (The Human Metabolome Database): | HMDB35250 |
| FooDB: | FDB013910 |
| Export Tariff Code: | 2912.29.5000 |
| Typical G.C. | |
| VCF-Online: | VCF Volatile Compounds in Food |
| ChemSpider: | View |
Potential Blenders and core components note
Potential Uses:
| amber | FR | |
| balsam | FR | |
| berry | FR | |
| cinnamon | FR | |
| dry | ||
| hawthorn | FR | |
| hay new mown hay | FR | |
| lime | FR | |
| melon | FR | |
| mint | FR | |
| myrtle oil replacer | FR | |
| oral care agents | ||
| spice | FR | |
| woody | FR |
Occurrence (nature, food, other): note
| amomum testaceum ridl. fruit oil malaysia @ 12.70% Data GC Search Trop Picture | |
| artemisia campestris spp. glutinosa flower oil italy @ 0.40% Data GC Search Trop Picture | |
| artemisia variabilis flower oil italy @ trace% Data GC Search Trop Picture | |
| bay laurel leaf Search Trop Picture | |
| boldo leaf oil italy @ trace% Data GC Search Trop Picture | |
| cardamom fruit oil Search Trop Picture | |
| celery seed Search Trop Picture | |
| chamomile garden chamomile plant Search Trop Picture | |
| chamomile oil @ 0.67% Data GC Search Picture | |
| cistus oil @ 0.9% Data GC Search Trop Picture | |
| coriander seed oil Search Trop Picture | |
| coriander seed oil cuba @ 0.16% Data GC Search Trop Picture | |
| cumin seed Search Trop Picture | |
| cypress cone oil egypt @ 0.1% Data GC Search Trop Picture | |
| cypress oil @ trace% Data GC Search Trop Picture | |
| eucalyptus Search PMC Picture | |
| eucalyptus globulus pseudoglobulus oil @ 0.11% Data GC Search Trop Picture | |
| ginger rhizome Search Trop Picture | |
| ginger rhizome oil Search Trop Picture | |
| horsemint shoot Search Trop Picture | |
| hyssop shoot Search Trop Picture | |
| labdanum leaf oil @ 0.50% Data GC Search Trop Picture | |
| labdanum oil @ 1.35% Data GC Search Trop Picture | |
| laurel leaf oil turkey @ 0.40% Data GC Search Trop Picture | |
| layana oil kenya @ trace% Data GC Search Trop Picture | |
| lemon verbena oil morocco @ 0.2% Data GC Search Trop Picture | |
| mint Search Trop Picture | |
| nepeta betonicifolia c.a. meyer oil turkey @ 0.20% Data GC Search Trop Picture | |
| nepeta denudata benth. oil iran @ 3.40% Data GC Search Trop Picture | |
| parsley leaf oil @ 0.05% Data GC Search Trop Picture | |
| pepper black pepper fruit Search Trop Picture | |
| pepper black pepper seed Search Trop Picture | |
| peppermint leaf Search Trop Picture | |
| petitgrain sweet oil @ 0.50% Data GC Search Picture | |
| peucedanum petiolare (dc) boiss oil iran @ 0.20% Data GC Search Trop Picture | |
| pteronia oil @ 0.50% Data GC Search Trop Picture | |
| salvia officinalis seed oil tunisia @ 0.55% Data GC Search Trop Picture | |
| santolina oil @ 3.82% Data GC Search Trop Picture | |
| satureja viminea l. oil costa rica @ 0.20% Data GC Search Trop Picture | |
| spearmint oil Search Trop Picture | |
| sunflower flower Search Trop Picture | |
| tansy oil marocco @ 0.10% Data GC Search Trop Picture | |
| wormwood flower oil annus america @ 0.40% Data GC Search Trop Picture | |
| wormwood leaf oil annus america @ 0.20-0.50% Data GC Search Trop Picture | |
| wormwood oil annus france @ trace-0.42% Data GC Search Trop Picture | |
| wormwood oil italy @ 0.1% Data GC Search Trop Picture | |
| yarrow leaf oil @ 0.60% Data GC Search Trop Picture | |
| yarrow oil @ 0.80% Data GC Search Trop Picture |
Synonyms:
| benihinal | |
| bicyclo[3.1.1]hept-2-ene-2-carboxaldehyde, 6,6-dimethyl- | |
| 6,6- | dimethyl bicyclo(3.1.1)hept-2-ene-2-carboxaldehyde |
| 6,6- | dimethyl-2-norpinene-2-carboxaldehyde |
| 6,6- | dimethylbicyclo(3.1.1)hept-2-ene-2-carboxaldehyde |
| 6,6- | dimethylbicyclo[3.1.1]hept-2-ene-2-carbaldehyde |
| 6,6- | dimethylbicyclo[3.1.1]hept-2-ene-2-carboxaldehyde |
| 7,7- | dimethylbicyclo[3.1.1]hept-3-ene-4-carbaldehyde |
| 2- | formyl-6,6-dimethyl bicyclo(3.1.1)hept-2-ene |
| 2- | formyl-6,6-dimethyl-2-norpinene |
| 2- | formyl-6,6-dimethylbicyclo(3.1.1)hept-2-ene |
| 2- | formyl-6,6-dimethylbicyclo[3.1.1]hept-2-ene (myrtenal) |
| (±)- | myrtenal |
| pin-2-ene-1-carbaldehyde | |
| 2-nor | pinene-2-carboxaldehyde, 6,6-dimethyl- |






