|
Category: flavoring agents
US / EU / FDA / JECFA / FEMA / FLAVIS / Scholar / Patent Information:
Physical Properties:
| Assay: | 95.00 to 100.00 %
|
| Food Chemicals Codex Listed: | No |
| Specific Gravity: | 1.24300 @ 25.00 °C.
|
| Refractive Index: | 1.56200 @ 20.00 °C.
|
| Melting Point: | 32.00 to 35.00 °C. @ 760.00 mm Hg
|
| Boiling Point: | 114.00 to 116.00 °C. @ 1.00 mm Hg
|
| Boiling Point: | 291.00 to 292.00 °C. @ 760.00 mm Hg
|
| Vapor Pressure: | 0.001000 mmHg @ 25.00 °C. (est) |
| Flash Point: | 175.00 °F. TCC ( 79.44 °C. )
|
| logP (o/w): | -0.778 (est) |
| Soluble in: |
| | alcohol | | | water, 3.643e+005 mg/L @ 25 °C (est) |
| Insoluble in: |
| | water |
Organoleptic Properties:
| |
| Odor Type: fatty |
| |
| | fatty buttery musty waxy caramellic |
Odor Description:
at 0.10 % in dipropylene glycol. | fatty buttery musty waxy caramellic |
| |
| |
| Flavor Type: herbal |
| |
| | herbal hay tobacco |
Taste Description:
| herbal hay tobacco |
| |
| Odor and/or flavor descriptions from others (if found). |
| |
| |
Cosmetic Information:
Suppliers:
| Alfa Biotechnology |
| For experimental / research use only. |
| 5-Hydroxymethylfurfural 98%
|
| Ambles Nature et Chimie |
| 5-HYDROXYMETHYL FURFURAL
|
| AVA Biochem |
| 5-Hydroxymethylfurfural
Odor: characteristic Use: Among the organic compounds that can be obtained from biomass, 5-Hydroxymethylfurfural (5-HMF) is one of the most interesting ones.
5-HMF is an organic compound derived from dehydration of certain sugars (hexoses). This yellow, low-melting solid is highly water-soluble. The molecule consists of a furan ring, containing both aldehyde and alcohol functional groups. HMF as natural substance has been identified in a wide variety of heat-processed foods including milk, fruit juices, spirits, honey, etc. HMF, which is derived from cellulose, is a potential "carbon-neutral" feedstock for a number of chemical substances.
AVA Biochem offers 5-HMF in various purities in crystalline form or in aqueous solution to its customers in the research and fine chemical industry. |
| Beijing Lys Chemicals |
| 5-(Hydroxymethyl)furfural
|
| BOC Sciences |
| For experimental / research use only. |
| 5-Hydroxymethylfurfural
|
| Connect Chemicals |
| 5-(Hydroxymethyl)furan-2-carbaldehyde
|
| ECSA Chemicals |
| 5-HYDROXYMETHYL-FURFURAL
|
| ECSA TRADE THE MOST UPDATED FINANCIAL PUBLICATION ON THE WORLD OF CHEMISTRY |
| Endeavour Specialty Chemicals |
| 5-(Hydroxymethyl)furfural
|
| Speciality Chemical Product Groups |
| Jalor-Chem |
| For experimental / research use only. |
| 5-Hydroxymethylfurfural
|
| Jinan Enlighten Chemical Technology(Wutong Aroma ) |
| 5-Hydroxymethyl furfural Kosherk
|
| M&U International |
| 5-HYDROXYMETHYL FURFURAL, Kosher
|
| Penta International |
| 5-(HYDROXYMETHYL) FURFURAL
|
| R C Treatt & Co Ltd |
| 5-(Hydroxymethyl)furfural
Kosher |
| Robinson Brothers |
| 5-(Hydroxymethyl)furfural F&F
|
| https://www.robinsonbrothers.uk/chemistry-competences |
| Santa Cruz Biotechnology |
| For experimental / research use only. |
| 5-Hydroxymethyl-2-furaldehyde
|
| Sigma-Aldrich |
| 5-(Hydroxymethyl)furfural, ≥99%, FG
Odor: butter; caramel; musty |
| Certified Food Grade Products |
| Sunaux International |
| 5-Hydroxymethyl Furfural
|
| Synerzine |
| 5-(Hydroxymethyl) Furfural
|
| TCI AMERICA |
| For experimental / research use only. |
| 5-Hydroxymethyl-2-furaldehyde (stabilized with Water) >95.0%(GC)
|
| Tengzhou Jitian Aroma Chemiclal |
| 5-Hydroxymethyl Furfural
|
| Tianjin Danjun International |
| 5-(Hydroxymethyl)furfural
|
| United International |
| 5-Hydroxymethyl furfural
|
| WholeChem |
| 5-(Hydroxymethyl)furfural
|
Safety Information:
| Preferred SDS: View |
| European information : |
| Most important hazard(s): | | Xi N - Irritant, Dangerous for the environment. |
R 36/37/38 - Irritating to eyes, respiratory system, and skin.
R 51/53 - Toxic to aquatic organisms, may cause long-term adverse effects in the aquatic environment.
S 02 - Keep out of the reach of children.
S 24/25 - Avoid contact with skin and eyes.
S 26 - In case of contact with eyes, rinse immediately with plenty of water and seek medical advice.
S 36 - Wear suitable protective clothing.
S 61 - Avoid release to the environment. Refer to special instructions/safety data sheet.
|
| |
| Hazards identification |
| |
| Classification of the substance or mixture |
| GHS Classification in accordance with 29 CFR 1910 (OSHA HCS) |
| None found. |
| GHS Label elements, including precautionary statements |
| |
| Pictogram | |
| |
| Hazard statement(s) |
| None found. |
| Precautionary statement(s) |
| None found. |
| Oral/Parenteral Toxicity: |
oral-rat LD50 3100 mg/kg
(Simonyan, 1969)
gavage-rat LD50 [sex: M,F] 2500 mg/kg
(Warf Institute, 1977)
oral-mouse LD50 1910 mg/kg
(Simonyan, 1969)
oral-mouse LD50 > 2000 mg/kg
(Czok, 1970)
oral-rat LD50 2500 mg/kg
BEHAVIORAL: CONVULSIONS OR EFFECT ON SEIZURE THRESHOLD
National Technical Information Service. Vol. OTS0544683
|
| Dermal Toxicity: |
|
Not determined
|
| Inhalation Toxicity: |
|
Not determined
|
Safety in Use Information:
| Category: | flavoring agents |
| Recommendation for 5-hydroxymethyl furfural usage levels up to: | | | not for fragrance use.
|
| |
| Maximised Survey-derived Daily Intakes (MSDI-EU): | 0.39 (μg/capita/day) |
| Modified Theoretical Added Maximum Daily Intake (mTAMDI): | 1600 (μg/person/day) |
| Threshold of Concern: | 540 (μg/person/day) |
| Structure Class: | II |
| |
| Food categories according to Commission Regulation EC No. 1565/2000 (EC, 2000) in FGE.06 (EFSA, 2002a). According to the Industry the "normal" use is defined as the average of reported usages and "maximum use" is defined as the 95th percentile of reported usages (EFSA, 2002i). |
| Note: mg/kg = 0.001/1000 = 0.000001 = 1/1000000 = ppm. |
| | average usage mg/kg | maximum usage mg/kg |
| Dairy products, excluding products of category 02.0 (01.0): | 3.00000 | 15.00000 |
| Fats and oils, and fat emulsions (type water-in-oil) (02.0): | 2.00000 | 10.00000 |
| Edible ices, including sherbet and sorbet (03.0): | 3.00000 | 15.00000 |
| Processed fruit (04.1): | 2.00000 | 10.00000 |
| Processed vegetables (incl. mushrooms & fungi, roots & tubers, pulses and legumes), and nuts & seeds (04.2): | - | - |
| Confectionery (05.0): | 4.00000 | 20.00000 |
| Chewing gum (05.0): | - | - |
| Cereals and cereal products, incl. flours & starches from roots & tubers, pulses & legumes, excluding bakery (06.0): | 2.00000 | 10.00000 |
| Bakery wares (07.0): | 5.00000 | 25.00000 |
| Meat and meat products, including poultry and game (08.0): | 1.00000 | 5.00000 |
| Fish and fish products, including molluscs, crustaceans and echinoderms (MCE) (09.0): | 1.00000 | 5.00000 |
| Eggs and egg products (10.0): | - | - |
| Sweeteners, including honey (11.0): | - | - |
| Salts, spices, soups, sauces, salads, protein products, etc. (12.0): | 2.00000 | 10.00000 |
| Foodstuffs intended for particular nutritional uses (13.0): | 3.00000 | 15.00000 |
| Non-alcoholic ("soft") beverages, excl. dairy products (14.1): | 2.00000 | 10.00000 |
| Alcoholic beverages, incl. alcohol-free and low-alcoholic counterparts (14.2): | 4.00000 | 20.00000 |
| Ready-to-eat savouries (15.0): | 5.00000 | 25.00000 |
| Composite foods (e.g. casseroles, meat pies, mincemeat) - foods that could not be placed in categories 01.0 - 15.0 (16.0): | 2.00000 | 10.00000 |
Safety References:
| European Food Safety Athority(EFSA): | Flavor usage levels; Subacute, Subchronic, Chronic and Carcinogenicity Studies; Developmental / Reproductive Toxicity Studies; Genotoxicity Studies... |
| European Food Safety Authority (EFSA) reference(s): |
Opinion of the Scientific Panel on food additives, flavourings, processing aids and materials in contact with food (AFC) related to Flavouring Group Evaluation 13 (FGE.13); Furfuryl and furan derivatives with and without additional side-chain substituents and heteroatoms from chemical group 14 (Commission Regulation (EC) No 1565/2000 of 18
View page or View pdf |
Flavouring Group Evaluation 66 (FGE.66)[1]:Consideration of furfuryl alcohol and related flavouring substances evaluated by JECFA (55th meeting) structurally related to Furfuryl and furan derivatives with and without additional side chain substituents and heteroatoms evaluated by EFSA in FGE.13 (2005)
View page or View pdf |
Scientific Opinion on Flavouring Group Evaluation 13Rev1: Furfuryl and furan derivatives with and without additional side-chain substituents and heteroatoms from chemical group 14
View page or View pdf |
Consideration of sulfur-substituted furan derivatives used as flavouring agents evaluated by JECFA (59th meeting) structurally related to a subgroup of substances within the group of “Furfuryl and furan derivatives with and without additional side-chain substituents and heteroatoms from chemical group 14” evaluated by EFSA in FGE.13Rev1 (2009)
View page or View pdf |
Flavouring Group Evaluation 67 (FGE.67): Consideration of 40 furan-substituted aliphatic hydrocarbons, alcohols, aldehydes, ketones, carboxylic acids and related esters, sulfides, disulfides and ethers evaluated by JECFA at the 65th meeting (JECFA, 2006b) and re-evaluated at the 69th meeting (JECFA, 2009c)
View page or View pdf |
Scientific Opinion on Flavouring Group Evaluation 13, Revision 2 (FGE.13Rev2): Furfuryl and furan derivatives with and without additional side-chain substituents and heteroatoms from chemical group 14
View page or View pdf |
Scientific Opinion on Flavouring Group Evaluation 67, Revision 1 (FGE.67Rev.1): Consideration of 40 furan-substituted aliphatic hydrocarbons, alcohols, aldehydes, ketones, carboxylic acids and related esters, sulfides, disulfides and ethers evaluated by JECFA at the 65th meeting (JECFA, 2006b) and re-evaluated at the 69th meeting (JECFA, 2009c)
View page or View pdf |
Extensive literature search on grayanotoxins and 5-hydroxymethylfurfural
View page or View pdf |
Scientific Opinion on Flavouring Group Evaluation 13 Revision 3 (FGE.13Rev3): furfuryl and furan derivatives with and without additional side-chain substituents and heteroatoms from chemical group 14
View page or View pdf |
| EPI System: | View |
| Chemical Carcinogenesis Research Information System: | Search |
| AIDS Citations: | Search |
| Cancer Citations: | Search |
| Toxicology Citations: | Search |
| EPA Substance Registry Services (TSCA): | 67-47-0 |
| EPA ACToR: | Toxicology Data |
| EPA Substance Registry Services (SRS): | Registry |
| Laboratory Chemical Safety Summary : | 237332 |
| National Institute of Allergy and Infectious Diseases: | Data |
| WGK Germany: | 2 |
| | 5-(hydroxymethyl)furan-2-carbaldehyde |
| Chemidplus: | 0000067470 |
| RTECS: | LT7031100 for cas# 67-47-0 |
References:
Other Information:
Potential Blenders and core components note
| |
| For Odor |
| acidic |
| | cyclohexyl acetic acid | FL/FR |
| aldehydic |
| | dodecanal (aldehyde C-12 lauric) | FL/FR |
| balsamic |
| 2- | acetyl furan | FL/FR |
| buttery |
| | acetoin propylene glycol acetal | FL/FR |
| | acetyl propionyl | FL/FR |
| 3,4- | hexane dione | FL/FR |
| 2-oxo | butyric acid | FL/FR |
| | caramel pentadione | FL/FR |
| | cyclotene | FL/FR |
| | cyclotene hydrate | FL/FR |
| alpha,alpha- | dimethyl anisyl acetone | FL/FR |
| | ethyl 2-hydroxy-2-methyl butyrate | FL/FR |
| | ethyl cyclopentenolone | FL/FR |
| | ethyl maltol | FL/FR |
| 5- | ethyl-2,3,4,5-tetramethyl-2-cyclohexen-1-one | FL/FR |
| | fenugreek absolute | FL/FR |
| | immortelle absolute | FL/FR |
| | levulinic acid | FL/FR |
| | maltol | FL/FR |
| | maltyl propionate | FL/FR |
| | maple furanone | FL/FR |
| | menthone lactone | FL/FR |
| 5- | methyl furfural | FL/FR |
| | rosefuran | FL/FR |
| | shoyu furanone | FL/FR |
| | strawberry furanone | FL/FR |
| | strawberry furanone acetate | FL/FR |
| | toffee furanone | FL/FR |
| coconut |
| gamma- | heptalactone | FL/FR |
| creamy |
| gamma- | butyrolactone | FL/FR |
| delta- | tridecalactone | FL/FR |
| ethereal |
| | ethyl 4-pentenoate | FL/FR |
| | ethyl pyruvate | FL/FR |
| fatty |
| | allyl octanoate | FL/FR |
| (Z)- | dairy lactone | FL/FR |
| 2,4- | decadien-1-ol | FL/FR |
| (E)-2- | decen-1-ol | FL/FR |
| 7- | decen-4-olide | FL/FR |
| (E)-2- | decenal | FL/FR |
| | ethyl undecylenate | FL/FR |
| | methyl 10-undecenoate | FL/FR |
| 4- | methyl octanoic acid | FL/FR |
| (Z)-2- | nonenal | CS |
| | octanoic acid | FL/FR |
| (Z)- | oleic acid | FL/FR |
| fermented |
| | ethyl (E)-2-crotonate | FL/FR |
| floral |
| | benzyl lactate | FL/FR |
| | jasmin absolute (from pommade) | FL/FR |
| fruity |
| | allyl cyclohexyl propionate | FL/FR |
| (S)-delta- | decalactone | FL/FR |
| | ethyl hexanoate | FL/FR |
| (E)- | ethyl tiglate | FL/FR |
| 2- | methyl butyl butyrate | FL/FR |
| | tetrahydrofurfuryl acetate | FL/FR |
| green |
| iso | amyl 3-(2-furan) propionate | FL/FR |
| | manzanate (Givaudan) | FL/FR |
| herbal |
| | butyl levulinate | FL/FR |
| | clary sage oil france | FL/FR |
| | salvia sclarea oil | FL/FR |
| musty |
| 3- | acetyl-2,5-dimethyl furan | FL/FR |
| nutty |
| 2- | acetyl-3,5(or 6)-dimethyl pyrazine | FL/FR |
| 2,3- | dimethyl pyrazine | FL/FR |
| | nutty cyclohexenone | FL/FR |
| phenolic |
| 4- | methyl-2,6-dimethoxyphenol | FL/FR |
| soapy |
| | benzyl laurate | FL/FR |
| | ethyl undecanoate | FL/FR |
| | methyl eugenol | FR |
| (E)- | tiglic acid | FL/FR |
| sweet |
| | vanilla oleoresin bali | FL/FR |
| tonka |
| 6- | amyl-alpha-pyrone | FL/FR |
| tropical |
| | genet absolute | FL/FR |
| vanilla |
| | ethyl vanillin | FL/FR |
| | vanillyl isobutyrate | FL/FR |
| vegetable |
| | tetrahydrofurfuryl alcohol | FL/FR |
| waxy |
| iso | amyl laurate | FL/FR |
| (Z)-5- | decen-1-ol | |
| (E)-2- | decen-1-yl acetate | FL/FR |
| 1- | dodecanol | FL/FR |
| | ethyl octanoate | FL/FR |
| | methyl myristate | FL/FR |
| | myristic acid | FL/FR |
| 2,4- | nonadien-1-ol | FL/FR |
| | palmitic acid | FR |
| | tetradecanal | FL/FR |
| |
| For Flavor |
| |
| No flavor group found for these |
| 2- | acetyl-3,4,5,6-tetrahydropyridine | FL |
| | allyl 2-furoate | FL |
| | benzyl disulfide | FL |
| | benzyl lactate | FL/FR |
| (S)-delta- | decalactone | FL/FR |
| (E)-2- | decen-1-ol | FL/FR |
| (Z)-5- | decen-1-ol | |
| (E)-2- | decen-1-yl acetate | FL/FR |
| 7- | decen-4-olide | FL/FR |
| 2,5- | diethyl tetrahydrofuran | FL |
| alpha,alpha- | dimethyl anisyl acetone | FL/FR |
| | ethyl 2-hydroxy-2-methyl butyrate | FL/FR |
| | ethyl 4-pentenoate | FL/FR |
| 5- | ethyl-2,3,4,5-tetramethyl-2-cyclohexen-1-one | FL/FR |
| | furfuryl acetone | FL |
| | furfuryl hexanoate | FL |
| | menthone lactone | FL/FR |
| | methyl myristate | FL/FR |
| 4,5- | octane dione | FL |
| | rosefuran | FL/FR |
|
| iso | amyl 3-(2-furan) propionate | FL/FR |
| acidic |
| | levulinic acid | FL/FR |
| bready |
| | mango furanone | FL |
| brown |
| 2-oxo | butyric acid | FL/FR |
| 5- | methyl furfural | FL/FR |
| | tetrahydrofurfuryl acetate | FL/FR |
| (E)- | tiglic acid | FL/FR |
| burnt |
| | furfuryl alcohol | FL |
| buttery |
| | acetoin propylene glycol acetal | FL/FR |
| | diacetyl | FL |
| 3,4- | hexane dione | FL/FR |
| caramellic |
| | caramel dione | FL |
| | caramel furanone | FL |
| | caramel pentadione | FL/FR |
| | cyclotene | FL/FR |
| | cyclotene hydrate | FL/FR |
| | ethyl maltol | FL/FR |
| 5- | ethyl-3,4,5,6-tetramethyl cyclohexen-2-one | FL |
| | fenugreek absolute | FL/FR |
| | maltol | FL/FR |
| | maltyl propionate | FL/FR |
| | maple furanone | FL/FR |
| 3- | methyl butyl 2-furyl butyrate | FL |
| | shoyu furanone | FL/FR |
| | strawberry furanone | FL/FR |
| | strawberry furanone acetate | FL/FR |
| | toffee furanone | FL/FR |
| coffee |
| 2- | thiophene thiol | FL |
| cooling |
| | manzanate (Givaudan) | FL/FR |
| creamy |
| 6- | amyl-alpha-pyrone | FL/FR |
| delta- | tridecalactone | FL/FR |
| ethereal |
| | benzyl laurate | FL/FR |
| fatty |
| | allyl octanoate | FL/FR |
| iso | amyl laurate | FL/FR |
| (Z)- | dairy lactone | FL/FR |
| 2,4- | decadien-1-ol | FL/FR |
| | ethyl undecylenate | FL/FR |
| 4- | methyl octanoic acid | FL/FR |
| 2,4- | nonadien-1-ol | FL/FR |
| (E)-2- | octenoic acid | FL |
| (Z)- | oleic acid | FL/FR |
| | tetradecanal | FL/FR |
| (E,E)-2,4- | undecadienal | FL |
| 2,4- | undecadienal | FL |
| floral |
| | jasmin absolute (from pommade) | FL/FR |
| fruity |
| | allyl cyclohexyl propionate | FL/FR |
| | ethyl (E)-2-octenoate | FL |
| | ethyl hexanoate | FL/FR |
| (E)- | ethyl tiglate | FL/FR |
| | furfuryl valerate | FL |
| 2- | methyl butyl butyrate | FL/FR |
| 2- | pentanoyl furan | FL |
| green |
| | immortelle absolute | FL/FR |
| hay |
| | genet absolute | FL/FR |
| herbal |
| | butyl levulinate | FL/FR |
| | clary sage oil france | FL/FR |
| | salvia sclarea oil | FL/FR |
| jammy |
| | ethyl cyclopentenolone | FL/FR |
| lactonic |
| gamma- | heptalactone | FL/FR |
| milky |
| gamma- | butyrolactone | FL/FR |
| musty |
| | ethyl (E)-2-crotonate | FL/FR |
| nutty |
| 2- | acetyl furan | FL/FR |
| 3- | acetyl-2,5-dimethyl furan | FL/FR |
| 2- | acetyl-3,5(or 6)-dimethyl pyrazine | FL/FR |
| 2,3- | dimethyl pyrazine | FL/FR |
| | nutty cyclohexenone | FL/FR |
| | peanut oxazole | FL |
| | tetrahydrofurfuryl alcohol | FL/FR |
| phenolic |
| 4- | methyl-2,6-dimethoxyphenol | FL/FR |
| rummy |
| | ethyl pyruvate | FL/FR |
| soapy |
| | dodecanal (aldehyde C-12 lauric) | FL/FR |
| 1- | dodecanol | FL/FR |
| | octanoic acid | FL/FR |
| sweet |
| | cyclohexyl acetic acid | FL/FR |
| dextro- | sorbitol | FL |
| | vanilla oleoresin bali | FL/FR |
| toasted |
| | acetyl propionyl | FL/FR |
| vanilla |
| | ethyl vanillin | FL/FR |
| | vanillyl isobutyrate | FL/FR |
| waxy |
| (E)-2- | decenal | FL/FR |
| | ethyl octanoate | FL/FR |
| | ethyl undecanoate | FL/FR |
| | furfuryl octanoate | FL |
| | methyl 10-undecenoate | FL/FR |
| | myristic acid | FL/FR |
| |
Potential Uses:
Occurrence (nature, food, other): noteSynonyms:
| 2- | furaldehyde, 5-(hydroxymethyl)- | | 2- | furaldehyde, 5-hydroxymethyl- | | 2- | furancarboxaldehyde, 5-(hydroxymethyl)- | | 5- | HMF | | 5- | hydroxymethyl furaldehyde | | | hydroxymethyl furfurole | | 5- | hydroxymethyl-2-formyl furan | | 5- | hydroxymethyl-2-formylfuran | | 5- | hydroxymethyl-2-furaldehyde | | 5- | hydroxymethyl-2-furancarboxaldehyde | | 5- | hydroxymethyl-2-furfural | | 2- | hydroxymethyl-5-furfural | | 5- | hydroxymethyl-furfural | | 5-( | hydroxymethyl) furfural | | 5-( | hydroxymethyl)-2-furaldehyde | | 5-( | hydroxymethyl)-2-furan carbonal | | 5-( | hydroxymethyl)-2-furan carboxaldehyde | | 5-( | hydroxymethyl)-2-furancarbonal | | 5-( | hydroxymethyl)-2-furancarboxaldehyde | | 5-( | hydroxymethyl)-2-furfural | | 5-( | hydroxymethyl)-furfural | | 5-( | hydroxymethyl)furan-2-carbaldehyde | | 5-( | hydroxymethyl)furan-2-carboxaldehyde | | 5-( | hydroxymethyl)furfural | | 5- | hydroxymethylfuraldehyde | | 5- | hydroxymethylfuran-2-aldehyde | | 5- | hydroxymethylfurfural | | | hydroxymethylfurfuralaldehyde | | | hydroxymethylfurfurole | | 5-oxy | methyl furfurole | | 5-oxy | methylfurfurole |
Articles:
| PubMed: | Non-targeted metabolomic reveals the effect of salt stress on global metabolite of halotolerant yeast Candida versatilis and principal component analysis. |
| PubMed: | Effect of roasting conditions on color and volatile profile including HMF level in sweet almonds (Prunus dulcis). |
| PubMed: | Honey collected from different floras of Chandigarh Tricity: a comparative study involving physicochemical parameters and biochemical activities. |
| PubMed: | Decrease of aged beer aroma by the reducing activity of brewing yeast. |
| PubMed: | Role of roasting conditions in the profile of volatile flavor chemicals formed from coffee beans. |
| PubMed: | 3-Hydroxy-4,5-dimethyl-2(5H)-furanone levels in fortified Madeira wines: relationship to sugar content. |
| PubMed: | Generation of Maillard compounds from inulin during the thermal processing of Agave tequilana Weber Var. azul. |
| PubMed: | Separation and determination of 4-methylimidazole, 2-methylimidazole and 5-hydroxymethylfurfural in beverages by amino trap column coupled with pulsed amperometric detection. |
| PubMed: | Tolerance of the Nanocellulose-Producing Bacterium Gluconacetobacter xylinus to Lignocellulose-Derived Acids and Aldehydes. |
| PubMed: | Advanced glycation end products, physico-chemical and sensory characteristics of cooked lamb loins affected by cooking method and addition of flavour precursors. |
| PubMed: | Non-targeted metabolomic reveals the effect of salt stress on global metabolite of halotolerant yeast Candida versatilis and principal component analysis. |
| PubMed: | Determination of caramel colorants' by-products in liquid foods by ultra-high-performance liquid chromatography-tandem mass spectrometry (UPLC-MS/MS). |
| PubMed: | Nutritional and physicochemical characteristic of commercial Spanish citrus juices. |
| PubMed: | Volatile profile of Madeira wines submitted to traditional accelerated ageing. |
| PubMed: | 5-HMF and carbohydrates content in stingless bee honey by CE before and after thermal treatment. |
| PubMed: | Properties and catalytic activity of magnetic and acidic ionic liquids: experimental and molecular simulation. |
| PubMed: | Validation of a HPLC method for determination of hydroxymethylfurfural in crude palm oil. |
| PubMed: | Tolerance of S. cerevisiae and Z. mobilis to inhibitors produced during dilute acid hydrolysis of soybean meal. |
| PubMed: | Enhancing the organoleptic and functional properties of jujube by a quick aging process. |
| PubMed: | Acrylamide, 5-hydroxymethylfurfural and N(ε)-carboxymethyl-lysine in coffee substitutes and instant coffees. |
| PubMed: | Kinetics of browning, phenolics, and 5-hydroxymethylfurfural in commercial sparkling wines. |
| PubMed: | Toxic compounds in honey. |
| PubMed: | Hydroxymethylfurfural: a possible emergent cause of honey bee mortality? |
| PubMed: | In vitro antioxidant and antiproliferative activities of 5-hydroxymethylfurfural. |
| PubMed: | Comparison of an HPTLC method with the Reflectoquant assay for rapid determination of 5-hydroxymethylfurfural in honey. |
| PubMed: | Application of a high-throughput process analytical technology metabolomics pipeline to Port wine forced ageing process. |
| PubMed: | New approaches to determination of HMF. |
| PubMed: | Developing an effective means to reduce 5-hydroxymethyl-2-furfural from caramel colour. |
| PubMed: | Isolation, structural elucidation, and biological evaluation of a 5-hydroxymethyl-2-furfural derivative, asfural, from enzyme-treated asparagus extract. |
| PubMed: | Impacts of selected dietary polyphenols on caramelization in model systems. |
| PubMed: | Physicochemical properties of the Harenna forest honey, Bale, Ethiopia. |
| PubMed: | Identification of 5-hydroxymethyl-2-furfural (5-HMF) in Cava sparkling wines by LC-DAD-MS/MS and NMR spectrometry. |
| PubMed: | Study of hydroxymethylfurfural and furfural formation in cakes during baking in different ovens, using a validated multiple-stage extraction-based analytical method. |
| PubMed: | Changes of antioxidant activity and formation of 5-hydroxymethylfurfural in honey during thermal and microwave processing. |
| PubMed: | Impact of flavour solvent (propylene glycol or triacetin) on vanillin, 5-(hydroxymethyl)furfural, 2,4-decadienal, 2,4-heptadienal, structural parameters and sensory perception of shortcake biscuits over accelerated shelf life testing. |
| PubMed: | Identification of bitter compounds in whole wheat bread. |
| PubMed: | Indirect competitive ELISA based on monoclonal antibody for the detection of 5-hydroxymethyl-2-furfural in milk, compared with HPLC. |
| PubMed: | Effect of concentration temperature on some bioactive compounds and antioxidant proprieties of date syrup. |
| PubMed: | Effects of extrusion, infrared and microwave processing on Maillard reaction products and phenolic compounds in soybean. |
| PubMed: | Industrially applicable strategies for mitigating acrylamide, furan, and 5-hydroxymethylfurfural in food. |
| PubMed: | Effect of 5-hydroxymethylfurfural derived from processed Cornus officinalis on the prevention of high glucose-induced oxidative stress in human umbilical vein endothelial cells and its mechanism. |
| PubMed: | Congeners in sugar cane spirits aged in casks of different woods. |
| PubMed: | Rapid determination of 5-hydroxymethylfurfural by DART ionization with time-of-flight mass spectrometry. |
| PubMed: | Short communication: possible mechanism for inhibiting the formation of polymers originated from 5-hydroxymethyl-2-furaldehyde by sulfite groups in the dairy thermal process. |
| PubMed: | Jujube honey from China: physicochemical characteristics and mineral contents. |
| PubMed: | Physicochemical and antioxidant properties of Malaysian honeys produced by Apis cerana, Apis dorsata and Apis mellifera. |
| PubMed: | Reactivity of thermally treated α-dicarbonyl compounds. |
| PubMed: | Untangling the chemistry of port wine aging with the use of GC-FID, multivariate statistics, and network reconstruction. |
| PubMed: | Thermal stability of anthocyanins and colourless phenolics in pomegranate (Punica granatum L.) juices and model solutions. |
| PubMed: | Determination of metabolites of 5-hydroxymethylfurfural in human urine after oral application. |
| PubMed: | Impact of cooking and handling conditions on furanic compounds in breaded fish products. |
| PubMed: | Effect of adulteration versus storage on volatiles in unifloral honeys from different floral sources and locations. |
| PubMed: | A comparison of different dilute solution explosions pretreatment for conversion of distillers' grains into ethanol. |
| PubMed: | Controlling the Maillard reaction by reactant encapsulation: sodium chloride in cookies. |
| PubMed: | Physicochemical and antioxidant properties of Bangladeshi honeys stored for more than one year. |
| PubMed: | Reporter gene mutation in the livers of gpt delta mice treated with 5-(hydroxymethyl)-2-furfural, a contaminant of various foods. |
| PubMed: | Okara promoted acrylamide and carboxymethyl-lysine formation in bakery products. |
| PubMed: | Short communication: simultaneous analysis of reducing sugars and 5-hydroxymethyl-2-furaldehyde at a low concentration by high performance anion exchange chromatography with electrochemical detector, compared with HPLC with refractive index detector. |
| PubMed: | Identification and mode of action of 5-hydroxymethyl-2-furfural (5-hmf) and 1-methyl-1,2,3,4-tetrahydro-β-carboline-3-carboxylic acid (MTCA) as potent xanthine oxidase inhibitors in vinegars. |
| PubMed: | Riboflavin and lumichrome in Dalmatian sage honey and other unifloral honeys determined by LC-DAD technique. |
| PubMed: | Development and validation of an HPLC method to determine metabolites of 5-hydroxymethylfurfural (5-HMF). |
| PubMed: | Volatile constituents of roasted tigernut oil (Cyperus esculentus L.). |
| PubMed: | Micellar electrokinetic chromatography method for the simultaneous determination of furanic compounds in honey and vegetable oils. |
| PubMed: | In depth study of acrylamide formation in coffee during roasting: role of sucrose decomposition and lipid oxidation. |
| PubMed: | Determination of very low levels of 5-(hydroxymethyl)-2-furaldehyde (HMF) in natural honey: comparison between the HPLC technique and the spectrophotometric white method. |
| PubMed: | 1,2-dicarbonyl compounds in commonly consumed foods. |
| PubMed: | Mutagenicity of 5-hydroxymethylfurfural in V79 cells expressing human SULT1A1: identification and mass spectrometric quantification of DNA adducts formed. |
| PubMed: | Effect of roasting conditions on color and volatile profile including HMF level in sweet almonds (Prunus dulcis). |
| PubMed: | Survey of 1,2-dicarbonyl compounds in commercial honey of different floral origin. |
| PubMed: | Effect of temperature and some added compounds on the stability of blood orange marmalade. |
| PubMed: | Kinetics of 3-deoxy-D-erythro-hexos-2-ulose in unifloral honeys. |
| PubMed: | Study of 5-hydroxymethylfurfural and its metabolite 5-sulfooxymethylfurfural on induction of colonic aberrant crypt foci in wild-type mice and transgenic mice expressing human sulfotransferases 1A1 and 1A2. |
| PubMed: | Optimization of furfural and 5-hydroxymethylfurfural production from wheat straw by a microwave-assisted process. |
| PubMed: | Simultaneous quantitation of 2-acetyl-4-tetrahydroxybutylimidazole, 2- and 4-methylimidazoles, and 5-hydroxymethylfurfural in beverages by ultrahigh-performance liquid chromatography-tandem mass spectrometry. |
| PubMed: | Analysis of roasted and unroasted Pistacia terebinthus volatiles using direct thermal desorption-GCxGC-TOF/MS. |
| PubMed: | The potential of biodetoxification activity as a probiotic property of Lactobacillus reuteri. |
| PubMed: | Degradation of 5-hydroxymethylfurfural during yeast fermentation. |
| PubMed: | Maillard reaction and protein cross-linking in relation to the solubility of milk powders. |
| PubMed: | Intestinal carcinogenesis of two food processing contaminants, 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine and 5-hydroxymethylfurfural, in transgenic FVB min mice expressing human sulfotransferases. |
| PubMed: | Development of an enzyme-linked immunosorbent assay for the determination of 5-hydroxymethyl-2-furfural in food. |
| PubMed: | Biological pretreatment with a cellobiose dehydrogenase-deficient strain of Trametes versicolor enhances the biofuel potential of canola straw. |
| PubMed: | Mead production: tradition versus modernity. |
| PubMed: | Thermal decomposition of 5-(hydroxymethyl)-2-furaldehyde (HMF) and its further transformations in the presence of glycine. |
| PubMed: | Hydroxymethyl-substituted furans: mutagenicity in Salmonella typhimurium strains engineered for expression of various human and rodent sulphotransferases. |
| PubMed: | Identification of Hâ‚‚Oâ‚‚ as a major antimicrobial component in coffee. |
| PubMed: | Fructo-oligosaccharide production from inulin through partial citric or phosphoric acid hydrolyses. |
| PubMed: | 5-Hydroxymethylfurfural content in foodstuffs determined by micellar electrokinetic chromatography. |
| PubMed: | Fast quantitation of 5-hydroxymethylfurfural in honey using planar chromatography. |
| PubMed: | Kinetic study of the thermal hydrolysis of Agave salmiana for mezcal production. |
| PubMed: | Reversible and covalent binding of 5-(hydroxymethyl)-2-furaldehyde (HMF) with lysine and selected amino acids. |
| PubMed: | Vitamin C and sugar levels as simple markers for discriminating Spanish honey sources. |
| PubMed: | Characterization of a wild strain of Alicyclobacillus acidoterrestris: heat resistance and implications for tomato juice. |
| PubMed: | Effect of antibrowning agents on browning and intermediate formation in the glucose-glutamic acid model. |
| PubMed: | Toxicology and risk assessment of 5-Hydroxymethylfurfural in food. |
| PubMed: | Effects of aldehydes on the growth and lipid accumulation of oleaginous yeast Trichosporon fermentans. |
| PubMed: | Thermal degradation characteristics and antioxidant activity of fructose solution with heating temperature and time. |
| PubMed: | nematicidal carboxylic acids and aldehydes from Melia azedarach fruits. |
| PubMed: | Use of green rooibos (Aspalathus linearis) extract and water-soluble nanomicelles of green rooibos extract encapsulated with ascorbic acid for enhanced aspalathin content in ready-to-drink iced teas. |
| PubMed: | Characterization of artisanal honey produced on the Northwest of Portugal by melissopalynological and physico-chemical data. |
| PubMed: | Optimization of extraction of apple pomace phenolics with water by response surface methodology. |
| PubMed: | Determination of some chemical parameters and antimicrobial activity of traditional food: mesir paste. |
| PubMed: | High 5-hydroxymethylfurfural concentrations are found in Malaysian honey samples stored for more than one year. |
| PubMed: | Glucose reversion reaction kinetics. |
| PubMed: | Determination of furan precursors and some thermal damage markers in baby foods: ascorbic acid, dehydroascorbic acid, hydroxymethylfurfural and furfural. |
| PubMed: | Does the pelleting process affect the nutritive value of a pre-starter diet for suckling piglets? Ex vivo studies on mineral absorption. |
| PubMed: | Quantitative determination of caffeine, formic acid, trigonelline and 5-(hydroxymethyl)furfural in soluble coffees by 1H NMR spectrometry. |
| PubMed: | Decrease of aged beer aroma by the reducing activity of brewing yeast. |
| PubMed: | Analysis, distribution, and dietary exposure of glyoxal and methylglyoxal in cookies and their relationship with other heat-induced contaminants. |
| PubMed: | Sucrose: A prospering and sustainable organic raw material. |
| PubMed: | Estimation of dietary intake of 5-hydroxymethylfurfural and related substances from coffee to Spanish population. |
| PubMed: | In vitro antioxidant activity of 5-HMF isolated from marine red alga Laurencia undulata in free-radical-mediated oxidative systems. |
| PubMed: | Physico-chemical studies on adulteration of honey in Nigeria. |
| PubMed: | Genotoxic activities of the food contaminant 5-hydroxymethylfurfural using different in vitro bioassays. |
| PubMed: | Effect of L-asparaginase on acrylamide mitigation in a fried-dough pastry model. |
| PubMed: | Simultaneous determination of melamine and 5-hydroxymethylfurfural in milk by capillary electrophoresis with diode array detection. |
| PubMed: | Assessment of hydroxymethylfurfural intake in the Spanish diet. |
| PubMed: | [Studies on chemical constituents from bee-collected rape pollen]. |
| PubMed: | Role of roasting conditions in the profile of volatile flavor chemicals formed from coffee beans. |
| PubMed: | 5-Hydroxymethylfurfural and 5-sulfooxymethylfurfural increase adenoma and flat ACF number in the intestine of Min/+ mice. |
| PubMed: | Improved method to measure aldehyde adducts to N-terminal valine in hemoglobin using 5-hydroxymethylfurfural and 2,5-furandialdehyde as model compounds. |
| PubMed: | Identification of an interfering substrate in apple juice and improvement for determination of patulin with high-performance liquid chromatography analyses. |
| PubMed: | Determination of 5-hydroxymethylfurfural using derivatization combined with polymer monolith microextraction by high-performance liquid chromatography. |
| PubMed: | Conversion of the common food constituent 5-hydroxymethylfurfural into a mutagenic and carcinogenic sulfuric acid ester in the mouse in vivo. |
| PubMed: | Development and validation of an HPLC method to quantify 3,4-dideoxyglucosone-3-ene in peritoneal dialysis fluids. |
| PubMed: | Evaluation of the DNA damaging effect of the heat-induced food toxicant 5-hydroxymethylfurfural (HMF) in various cell lines with different activities of sulfotransferases. |
| PubMed: | Influence of roasting on the antioxidant activity and HMF formation of a cocoa bean model systems. |
| PubMed: | Wheat straw autohydrolysis: process optimization and products characterization. |
| PubMed: | Evaluation of antioxidant activity, colour and some nutritional characteristics of pomegranate (Punica granatum L.) juice and its sour concentrate processed by conventional evaporation. |
| PubMed: | Degradation of 5-hydroxymethylfurfural in honey. |
| PubMed: | Simple gas chromatographic method for furfural analysis. |
| PubMed: | Dietary exposure to 5-hydroxymethylfurfural from Norwegian food and correlations with urine metabolites of short-term exposure. |
| PubMed: | Quality evaluation of different honey samples produced in Peshawar valley. |
| PubMed: | Isotope labeling studies on the formation of 5-(hydroxymethyl)-2-furaldehyde (HMF) from sucrose by pyrolysis-GC/MS. |
| PubMed: | Changes in the chemical composition of reduced cooked musts during the heating process. |
| PubMed: | Impact of caramelization on the glass transition temperature of several caramelized sugars. Part II: Mathematical modeling. |
| PubMed: | Comparative study of pulsed electric field and thermal processing of apple juice with particular consideration of juice quality and enzyme deactivation. |
| PubMed: | Estimation of hydroxymethylfurfural availability in breakfast cereals. Studies in Caco-2 cells. |
| PubMed: | Liquid chromatography multi-stage mass spectrometry for the analysis of 5-hydroxymethylfurfural in foods. |
| PubMed: | Evolution of hydroxymethylfurfural content of honeys from different climates: influence of induced granulation. |
| PubMed: | Investigation of acrylamide in curries made from coconut milk. |
| PubMed: | SULT1C3, an orphan sequence of the human genome, encodes an enzyme activating various promutagens. |
| PubMed: | The effect of various drying techniques on apricot volatiles analysed using direct thermal desorption-GC-TOF/MS. |
| PubMed: | Determination of patulin in commercial apple juice by micellar electrokinetic chromatography. |
| PubMed: | The potential antimutagenic and antioxidant effects of Maillard reaction products used as "natural antibrowning" agents. |
| PubMed: | Formation of 5-hydroxymethyl-2-furfural (HMF) and 5-hydroxymethyl-2-furoic acid during roasting of coffee. |
| PubMed: | Inhibition of polyphenoloxidase activity by mixtures of heated cysteine derivatives with carbonyl compounds. |
| PubMed: | Isolation and identification of potential cancer chemopreventive agents from methanolic extracts of green onion (Allium cepa). |
| PubMed: | Maillard reaction indicators in diets usually consumed by adolescent population. |
| PubMed: | Analysis of 5-hydroxymethylfurfural in foods by gas chromatography-mass spectrometry. |
| PubMed: | Analysis of 5-hydroxymethylfurfual in coffee, dried fruits and urine. |
| PubMed: | Study on fluorescence of Maillard reaction compounds in breakfast cereals. |
| PubMed: | Relationship between acrylamide and thermal-processing indexes in commercial breakfast cereals: a survey of Spanish breakfast cereals. |
| PubMed: | Identification and urinary excretion of metabolites of 5-(hydroxymethyl)-2-furfural in human subjects following consumption of dried plums or dried plum juice. |
| PubMed: | Diets rich in Maillard reaction products affect protein digestibility in adolescent males aged 11-14 y. |
| PubMed: | Improved method for the determination of hydroxymethylfurfural in baby foods using liquid chromatography-mass spectrometry. |
| PubMed: | Analysis of amino acids and carbohydrates in green coffee. |
| PubMed: | 5-(Hydroxymethyl)-2-furfural: a selective inhibitor of DNA polymerase lambda and terminal deoxynucleotidyltransferase. |
| PubMed: | Browning of model orange juice solution: factors affecting the formation of decomposition products. |
| PubMed: | Amla (Emblica officinalis Gaertn.) extracts reduce oxidative stress in streptozotocin-induced diabetic rats. |
| PubMed: | Honey in combination with vacuum impregnation to prevent enzymatic browning of fresh-cut apples. |
| PubMed: | Effect of storage period under variable conditions on the chemical and physical composition and colour of Spanish refrigerated orange juices. |
| PubMed: | Single laboratory validation of a method for the determination of hydroxymethylfurfural in honey by using solid-phase extraction cleanup and liquid chromatography. |
| PubMed: | Quality evaluation of honey harvested from selected areas in Tanzania with special emphasis on hydroxymethyl furfural (HMF) levels. |
| PubMed: | 3-Hydroxy-4,5-dimethyl-2(5H)-furanone levels in fortified Madeira wines: relationship to sugar content. |
| PubMed: | A benzenoid from the stem of Acanthopanax senticosus. |
| PubMed: | 1H NMR studies on Italian balsamic and traditional balsamic vinegars. |
| PubMed: | Browning and decomposed products of model orange juice. |
| PubMed: | Influence of distillation system, oak wood type, and aging time on composition of cider brandy in phenolic and furanic compounds. |
| PubMed: | 2-Furoylmethyl amino acids and hydroxymethylfurfural as indicators of honey quality. |
| PubMed: | Influence of seasonal variation on kinetics of time temperature integrators for thermally processed milk. |
| PubMed: | Thermal decomposition of specifically phosphorylated D-glucoses and their role in the control of the Maillard reaction. |
| PubMed: | Kinetics of hydroxymethylfurfural, lactulose and furosine formation in milk with different fat content. |
| PubMed: | Phytochemical composition and antioxidant stability of fortified yellow passion fruit (Passiflora edulis). |
| PubMed: | Identification and quantification of antioxidant components of honeys from various floral sources. |
| PubMed: | Interactions between cyanidin 3-O-glucoside and furfural derivatives and their impact on food color changes. |
| PubMed: | Role of aldehydic derivatives in the condensation of phenolic compounds with emphasis on the sensorial properties of fruit-derived foods. |
| PubMed: | Antioxidant effects of isorhamnetin 3,7-di-O-beta-D-glucopyranoside isolated from mustard leaf (Brassica juncea) in rats with streptozotocin-induced diabetes. |
| PubMed: | Erythritol attenuates the diabetic oxidative stress through modulating glucose metabolism and lipid peroxidation in streptozotocin-induced diabetic rats. |
| PubMed: | Generation of Maillard compounds from inulin during the thermal processing of Agave tequilana Weber Var. azul. |
| PubMed: | Formation kinetics of hydroxymethylfurfural, lactulose and furosine in milk heated under isothermal and non-isothermal conditions. |
| PubMed: | Supercritical fluid extraction of 5-hydroxymethyl-2-furaldehyde from raisins. |
| PubMed: | Derivative spectrophotometric determination of 5-(hydroxymethyl)-2-furaldehyde (HMF) and furfural in Locust bean extract. |
| PubMed: | Effects of some hydrocolloids and water activity on nonenzymic browning of concentrated orange juice. |
| PubMed: | Study of the reactions between (+)-catechin and furfural derivatives in the presence or absence of anthocyanins and their implication in food color change. |
| PubMed: | Use of 2-furoylmethyl derivatives of GABA and arginine as indicators of the initial steps of maillard reaction in orange juice. |
| PubMed: | 5-Hydroxymethylfurfural: assessment of mutagenicity, DNA-damaging potential and reactivity towards cellular glutathione. |
| PubMed: | Effects of Ca(OH)(2) treatments ("overliming") on the composition and toxicity of bagasse hemicellulose hydrolysates. |
| PubMed: | Confirmation of patulin and 5-hydroxymethylfurfural in apple juice by gas chromatography/mass spectrometry. |
| PubMed: | Effect of hexanal and iron on color development in a glucose/phenylalanine model system. |
| PubMed: | Hydroxymethylfurfural and furosine reaction kinetics in tomato products. |
| PubMed: | Study of the formation mechanisms of some volatile compounds during the aging of sweet fortified wines. |
| PubMed: | Effects of L-cysteine and N-acetyl-L-cysteine on 4-hydroxy-2, 5-dimethyl-3(2H)-furanone (furaneol), 5-(hydroxymethyl)furfural, and 5-methylfurfural formation and browning in buffer solutions containing either rhamnose or glucose and arginine. |
| PubMed: | Phenolic constituents, furans, and total antioxidant status of distilled spirits. |
| PubMed: | Analysis of furanone, pyranone, and new heterocyclic colored compounds from sugar-glycine model Maillard systems. |
| PubMed: | Effect of selected aldehydes on the growth and fermentation of ethanologenic Escherichia coli. |
| PubMed: | Simultaneous determination of 5-hydroxymethylfurfural and patulin in apple juice by reversed-phase liquid chromatography. |
| PubMed: | Distribution and metabolism of (5-hydroxymethyl)furfural in male F344 rats and B6C3F1 mice after oral administration. |
| PubMed: | Rapid reversed-phase liquid chromatographic determination of patulin in apple juice. |
| PubMed: | Bioactivation of 5-hydroxymethyl-2-furaldehyde to an electrophilic and mutagenic allylic sulfuric acid ester. |
| PubMed: | Semiautomatic determination of furanic aldehydes in food and pharmaceutical samples by a stopped-flow injection analysis method. |
| PubMed: | Detection and determination of interfering 5-hydroxymethylfurfural in the analysis of caramel-coloured pharmaceutical syrups. |
| PubMed: | Toxicity potential of compounds found in parenteral solutions with rubber stoppers. |
| PubMed: | Reversed-phase high-performance liquid chromatographic method for the quantification of 5-hydroxymethylfurfural as the major degradation product of glucose in infusion fluids. |
| PubMed: | Influence of water activity and reaction temperature of ribose-lysine and glucose-lysine Maillard systems on mutagenicity, absorbance and content of furfurals. |
| PubMed: | [Formation of hydroxymethylfurfural during the storage and processing of food products]. |
| PubMed: | Desmutagenic effect of alpha-dicarbonyl and alpha-hydroxycarbonyl compounds against mutagenic heterocyclic amines. |
| PubMed: | Rapid and complete urinary elimination of [14C]-5-hydroxymethyl-2-furaldehyde administered orally or intravenously to rats. |
| PubMed: | High performance liquid chromatography of furfural and hydroxymethylfurfural in spirits and honey. |
| PubMed: | Hydroxymethylfurfural and honey adulteration. |
| PubMed: | [Hygienic significance of patulin in foods. 1. Analytical detection of patulin]. |
|
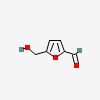 3D/inchi
3D/inchi














