|
Category: flavor and fragrance agents
US / EU / FDA / JECFA / FEMA / FLAVIS / Scholar / Patent Information:
Physical Properties:
| Appearance: | white to brown crystals (est) |
| Assay: | 97.00 to 100.00 %
|
| Food Chemicals Codex Listed: | No |
| Melting Point: | 126.00 to 133.00 °C. @ 760.00 mm Hg
|
| Boiling Point: | 215.00 to 217.00 °C. @ 760.00 mm Hg
|
| Acid Value: | 4.00 max. KOH/g
|
| Vapor Pressure: | 0.027000 mmHg @ 25.00 °C. (est) |
| Flash Point: | 207.00 °F. TCC ( 97.22 °C. )
|
| logP (o/w): | -0.611 (est) |
| Shelf Life: | 12.00 month(s) or longer if stored properly. |
| Storage: | store in cool, dry place in tightly sealed containers, protected from heat and light. |
| Soluble in: |
| | dipropylene glycol | | | propylene glycol | | | water, 4.739e+004 mg/L @ 25 °C (est) |
Organoleptic Properties:
Cosmetic Information:
Suppliers:
Safety Information:
| Preferred SDS: View |
| European information : |
| Most important hazard(s): | | Xn - Harmful. |
R 20/22 - Harmful by inhalation and if swallowed.
R 36/37/38 - Irritating to eyes, respiratory system, and skin.
S 02 - Keep out of the reach of children.
S 22 - Do not breath dust.
S 26 - In case of contact with eyes, rinse immediately with plenty of water and seek medical advice.
S 36/37/39 - Wear suitable clothing, gloves and eye/face protection.
|
| |
| Hazards identification |
| |
| Classification of the substance or mixture |
| GHS Classification in accordance with 29 CFR 1910 (OSHA HCS) |
| None found. |
| GHS Label elements, including precautionary statements |
| |
| Pictogram | |
| |
| Hazard statement(s) |
| None found. |
| Precautionary statement(s) |
| None found. |
| Oral/Parenteral Toxicity: |
|
Not determined
|
| Dermal Toxicity: |
|
Not determined
|
| Inhalation Toxicity: |
|
Not determined
|
Safety in Use Information:
| Category: | flavor and fragrance agents |
| RIFM Fragrance Material Safety Assessment: Search |
| IFRA Code of Practice Notification of the 49th Amendment to the IFRA Code of Practice |
| Recommendation for toffee furanone usage levels up to: | | | 0.1000 % in the fragrance concentrate.
|
| |
| Maximised Survey-derived Daily Intakes (MSDI-EU): | 5.60 (μg/capita/day) |
| Maximised Survey-derived Daily Intakes (MSDI-USA): | 0.07 (μg/capita/day) |
| Structure Class: | II |
| Use levels for FEMA GRAS flavoring substances on which the FEMA Expert Panel based its judgments that the substances are generally recognized as safe (GRAS). |
| The Expert Panel also publishes separate extensive reviews of scientific information on all FEMA GRAS flavoring substances and can be found at FEMA Flavor Ingredient Library |
| publication number: 12 |
| Click here to view publication 12 |
| | average usual ppm | average maximum ppm |
| baked goods: | - | - |
| beverages(nonalcoholic): | - | - |
| beverages(alcoholic): | - | - |
| breakfast cereal: | - | - |
| cheese: | - | - |
| chewing gum: | - | - |
| condiments / relishes: | - | 20.00000 |
| confectionery froastings: | - | - |
| egg products: | - | - |
| fats / oils: | - | 20.00000 |
| fish products: | - | 35.00000 |
| frozen dairy: | - | - |
| fruit ices: | - | - |
| gelatins / puddings: | - | 10.00000 |
| granulated sugar: | - | - |
| gravies: | - | 10.00000 |
| hard candy: | - | - |
| imitation dairy: | - | - |
| instant coffee / tea: | - | - |
| jams / jellies: | - | - |
| meat products: | - | 35.00000 |
| milk products: | - | - |
| nut products: | - | 20.00000 |
| other grains: | - | - |
| poultry: | - | 35.00000 |
| processed fruits: | - | - |
| processed vegetables: | - | - |
| reconstituted vegetables: | - | - |
| seasonings / flavors: | - | 1.00000 |
| snack foods: | - | 35.00000 |
| soft candy: | - | - |
| soups: | - | 10.00000 |
| sugar substitutes: | - | - |
| sweet sauces: | - | 20.00000 |
Safety References:
| European Food Safety Athority(EFSA): | Flavor usage levels; Subacute, Subchronic, Chronic and Carcinogenicity Studies; Developmental / Reproductive Toxicity Studies; Genotoxicity Studies... |
| European Food Safety Authority (EFSA) reference(s): |
Flavouring Group Evaluation 220: alpha,beta-Unsaturated ketones and precursors from chemical subgroup 4.4 of FGE.19: 3(2H)-Furanones
View page or View pdf |
Scientific Opinion on Flavouring Group Evaluation 220, Revision 1 (FGE.220Rev1): alpha,beta-Unsaturated ketones and precursors from chemical subgroup 4.4 of FGE.19: 3(2H)-Furanones
View page or View pdf |
Scientific Opinion on Flavouring Group Evaluation 99 (FGE.99): Consideration of furanone derivatives evaluated by the JECFA (63rd, 65th and 69th meetings)
View page or View pdf |
Scientific Opinion on Flavouring Group Evaluation 220, Revision 2 (FGE.220Rev1): a,▀-Unsaturated ketones and precursors from chemical subgroup 4.4 of FGE.19: 3(2H)-Furanones
View page or View pdf |
Scientific Opinion on Flavouring Group Evaluation 220 Revision 3 (FGE.220Rev3): Consideration of genotoxic potential for a,▀-unsaturated 3(2H)-Furanones from subgroup 4.4 of FGE.19
View page or View pdf |
Scientific Opinion on Flavouring Group Evaluation 99 Revision 1 (FGE.99Rev1): Consideration of furanone derivatives evaluated by the JECFA (63rd, 65th and 69th meetings)
View page or View pdf |
| EPI System: | View |
| Chemical Carcinogenesis Research Information System: | Search |
| AIDS Citations: | Search |
| Cancer Citations: | Search |
| Toxicology Citations: | Search |
| EPA Substance Registry Services (TSCA): | 19322-27-1 |
| EPA ACToR: | Toxicology Data |
| EPA Substance Registry Services (SRS): | Registry |
| Laboratory Chemical Safety Summary : | 4564493 |
| National Institute of Allergy and Infectious Diseases: | Data |
| WGK Germany: | 3 |
| | 4-hydroxy-5-methylfuran-3-one |
| Chemidplus: | 0019322271 |
References:
Other Information:
Potential Blenders and core components note
| |
| For Odor |
| acidic |
| acidic |
| | cyclohexyl acetic acid | FL/FR |
| balsamic |
| 2- | acetyl furan | FL/FR |
| | benzyl salicylate | FL/FR |
| | fir balsam absolute | FR |
| 3- | phenyl propyl alcohol | FL/FR |
| berry |
| | raspberry ketone | FL/FR |
| bready |
| | coffee furanone | FL/FR |
| buttery |
| | acetoin | FL/FR |
| | acetyl propionyl | FL/FR |
| 3,4- | hexane dione | FL/FR |
| 2-iso | butyl-3-methyl pyrazine | FL/FR |
| 2-oxo | butyric acid | FL/FR |
| | caramel pentadione | FL/FR |
| | coffee dione | FL/FR |
| | cyclotene | FL/FR |
| | cyclotene hydrate | FL/FR |
| | diethyl malate | FL/FR |
| alpha,alpha- | dimethyl anisyl acetone | FL/FR |
| | ethyl 2-hydroxy-2-methyl butyrate | FL/FR |
| | ethyl cyclopentenolone | FL/FR |
| | ethyl maltol | FL/FR |
| 5- | ethyl-2,3,4,5-tetramethyl-2-cyclohexen-1-one | FL/FR |
| | fenugreek absolute | FL/FR |
| | geranyl crotonate | FR |
| | immortelle absolute | FL/FR |
| | levulinic acid | FL/FR |
| | maltol | FL/FR |
| | maltyl isobutyrate | FL/FR |
| | maltyl propionate | FL/FR |
| | maple furanone | FL/FR |
| | menthone lactone | FL/FR |
| | mesitene lactone | FR |
| 5- | methyl furfural | FL/FR |
| iso | propenyl pyrazine | FL/FR |
| | rosefuran | FL/FR |
| | shoyu furanone | FL/FR |
| | strawberry furanone | FL/FR |
| | strawberry furanone solution | FL/FR |
| chocolate |
| 2,4,5- | trimethyl thiazole | FL/FR |
| citrus |
| blood | orange oil italy | FL/FR |
| coconut |
| gamma- | heptalactone | FL/FR |
| gamma- | octalactone | FL/FR |
| coumarinic |
| | tonka bean resinoid | FR |
| creamy |
| gamma- | butyrolactone | FL/FR |
| ethereal |
| | ethyl 4-pentenoate | FL/FR |
| | ethyl pyruvate | FL/FR |
| iso | valeraldehyde propylene glycol acetal | FL/FR |
| fermented |
| | ethyl (E)-2-crotonate | FL/FR |
| floral |
| beta- | damascenone | FL/FR |
| | ethyl phenyl acetate | FL/FR |
| | floral pyranol | FR |
| | heliotropin | FL/FR |
| | heliotropyl acetone | FL/FR |
| alpha- | hexyl cinnamaldehyde | FL/FR |
| | methyl dihydrojasmonate | FL/FR |
| | mimosa absolute france | FL/FR |
| | nerolidol | FL/FR |
| | ocean propanal | FL/FR |
| fruity |
| beta- | damascone | FL/FR |
| gamma- | decalactone | FL/FR |
| (E)- | ethyl tiglate | FL/FR |
| | osmanthus flower absolute | FL/FR |
| | tetrahydrofurfuryl acetate | FL/FR |
| gamma- | undecalactone (aldehyde C-14 (so-called)) | FL/FR |
| green |
| iso | amyl 3-(2-furan) propionate | FL/FR |
| | methyl octine carbonate | FL/FR |
| (E,Z)-2,6- | nonadien-1-ol | FL/FR |
| | violet leaf absolute | FL/FR |
| hay |
| | beeswax absolute | FL/FR |
| herbal |
| | butyl levulinate | FL/FR |
| honey |
| | methyl phenyl acetate | FL/FR |
| melon |
| | melon heptenal | FL/FR |
| (Z)-6- | nonenal | FL/FR |
| mossy |
| | veramoss (IFF) | FR |
| musty |
| 3- | acetyl-2,5-dimethyl furan | FL/FR |
| nutty |
| 2- | acetyl-3,5(or 6)-dimethyl pyrazine | FL/FR |
| 2,3- | dimethyl pyrazine | FL/FR |
| | nutty cyclohexenone | FL/FR |
| phenolic |
| 4- | methyl-2,6-dimethoxyphenol | FL/FR |
| popcorn |
| 2- | acetyl pyrazine | FL/FR |
| 2- | acetyl pyridine | FL/FR |
| powdery |
| para- | anisyl acetate | FL/FR |
| spicy |
| | cassia bark oil china | FL/FR |
| black | currant bud absolute | FL/FR |
| iso | eugenyl acetate | FL/FR |
| (E)- | tiglic acid | FL/FR |
| sugar |
| | cotton candy fragrance | FR |
| sweet |
| | vanilla oleoresin bali | FL/FR |
| tonka |
| | tonka bean absolute | FR |
| vanilla |
| | ethyl vanillin | FL/FR |
| | vanillin | FL/FR |
| | vanillyl isobutyrate | FL/FR |
| vegetable |
| | tetrahydrofurfuryl alcohol | FL/FR |
| woody |
| | santall | FR |
| |
| For Flavor |
| |
| No flavor group found for these |
| 2- | acetyl-3,4,5,6-tetrahydropyridine | FL |
| | allyl 2-furoate | FL |
| | benzyl disulfide | FL |
| | diethyl malate | FL/FR |
| 2,5- | diethyl tetrahydrofuran | FL |
| alpha,alpha- | dimethyl anisyl acetone | FL/FR |
| | ethyl 2-hydroxy-2-methyl butyrate | FL/FR |
| | ethyl 2,5-dimethyl-3-oxo-4(2H)-furyl carbonate | FL |
| | ethyl 4-pentenoate | FL/FR |
| 5- | ethyl-2,3,4,5-tetramethyl-2-cyclohexen-1-one | FL/FR |
| | furfuryl acetone | FL |
| | menthone lactone | FL/FR |
| | rosefuran | FL/FR |
|
| iso | amyl 3-(2-furan) propionate | FL/FR |
| beta- | damascone | FL/FR |
| acidic |
| | levulinic acid | FL/FR |
| dextro,laevo- | tartaric acid | FL |
| apple |
| (E,Z)-2,6- | nonadien-1-ol | FL/FR |
| balsamic |
| | benzyl salicylate | FL/FR |
| berry |
| | heliotropyl acetone | FL/FR |
| | raspberry ketone | FL/FR |
| bready |
| | mango furanone | FL |
| brown |
| | beeswax absolute | FL/FR |
| 2-oxo | butyric acid | FL/FR |
| 5- | methyl furfural | FL/FR |
| | tetrahydrofurfuryl acetate | FL/FR |
| (E)- | tiglic acid | FL/FR |
| burnt |
| | furfuryl alcohol | FL |
| iso | propenyl pyrazine | FL/FR |
| buttery |
| | diacetyl | FL |
| 3,4- | hexane dione | FL/FR |
| caramellic |
| | caramel dione | FL |
| | caramel furanone | FL |
| | caramel pentadione | FL/FR |
| | cyclotene | FL/FR |
| | cyclotene hydrate | FL/FR |
| | ethyl maltol | FL/FR |
| 5- | ethyl-3,4,5,6-tetramethyl cyclohexen-2-one | FL |
| | fenugreek absolute | FL/FR |
| | maltol | FL/FR |
| | maltyl propionate | FL/FR |
| | maple furanone | FL/FR |
| 3- | methyl butyl 2-furyl butyrate | FL |
| | shoyu furanone | FL/FR |
| | strawberry furanone | FL/FR |
| | strawberry furanone solution | FL/FR |
| cherry |
| | heliotropin | FL/FR |
| citrus |
| blood | orange oil italy | FL/FR |
| coffee |
| | coffee dione | FL/FR |
| 2- | thiophene thiol | FL |
| corn |
| 2- | acetyl pyridine | FL/FR |
| 2- | acetyl-2-thiazoline | FL |
| creamy |
| | acetoin | FL/FR |
| gamma- | undecalactone (aldehyde C-14 (so-called)) | FL/FR |
| dairy |
| | creme brulle coffee flavor | FL |
| fatty |
| 2,4- | undecadienal | FL |
| (E,E)-2,4- | undecadienal | FL |
| floral |
| | methyl dihydrojasmonate | FL/FR |
| | methyl phenyl acetate | FL/FR |
| | ocean propanal | FL/FR |
| fruity |
| para- | anisyl acetate | FL/FR |
| gamma- | decalactone | FL/FR |
| (E)- | ethyl tiglate | FL/FR |
| | furfuryl valerate | FL |
| | osmanthus flower absolute | FL/FR |
| 2- | pentanoyl furan | FL |
| iso | valeraldehyde propylene glycol acetal | FL/FR |
| green |
| 2-iso | butyl-3-methyl pyrazine | FL/FR |
| | immortelle absolute | FL/FR |
| | melon heptenal | FL/FR |
| | methyl octine carbonate | FL/FR |
| | nerolidol | FL/FR |
| (Z)-6- | nonenal | FL/FR |
| | violet leaf absolute | FL/FR |
| herbal |
| | butyl levulinate | FL/FR |
| 5- | hydroxymethyl furfural | FL |
| honey |
| | ethyl phenyl acetate | FL/FR |
| jammy |
| | ethyl cyclopentenolone | FL/FR |
| | maltyl isobutyrate | FL/FR |
| lactonic |
| gamma- | heptalactone | FL/FR |
| gamma- | octalactone | FL/FR |
| meaty |
| 2- | mercaptomethyl pyrazine | FL |
| milky |
| gamma- | butyrolactone | FL/FR |
| musty |
| | ethyl (E)-2-crotonate | FL/FR |
| nutty |
| 2- | acetyl furan | FL/FR |
| 3- | acetyl-2,5-dimethyl furan | FL/FR |
| 2- | acetyl-3,5(or 6)-dimethyl pyrazine | FL/FR |
| 3,5(6)- | cocoa pyrazine | FL |
| | coffee furanone | FL/FR |
| 2,3- | dimethyl pyrazine | FL/FR |
| | nutty cyclohexenone | FL/FR |
| | peanut oxazole | FL |
| | tetrahydrofurfuryl alcohol | FL/FR |
| 2,4,5- | trimethyl thiazole | FL/FR |
| phenolic |
| 4- | methyl-2,6-dimethoxyphenol | FL/FR |
| roasted |
| 2- | acetyl pyrazine | FL/FR |
| rummy |
| | ethyl pyruvate | FL/FR |
| spicy |
| | cassia bark oil china | FL/FR |
| black | currant bud absolute | FL/FR |
| iso | eugenyl acetate | FL/FR |
| 3- | phenyl propyl alcohol | FL/FR |
| sweet |
| | cyclohexyl acetic acid | FL/FR |
| dextro- | sorbitol | FL |
| | vanilla oleoresin bali | FL/FR |
| toasted |
| | acetyl propionyl | FL/FR |
| vanilla |
| | ethyl vanillin | FL/FR |
| | vanillin | FL/FR |
| | vanillyl isobutyrate | FL/FR |
| waxy |
| | furfuryl octanoate | FL |
| alpha- | hexyl cinnamaldehyde | FL/FR |
| | mimosa absolute france | FL/FR |
| woody |
| beta- | damascenone | FL/FR |
| |
Potential Uses:
Occurrence (nature, food, other): noteSynonyms:
| | chicory furaneol | | | chicory furanone | | | chicory furanone 5% in PG natural | | | chicory furanone 5% in PG synthetic | | | chicory furanone synthetic | | | furan-3(2H)-one, 4-hydroxy-5-methyl- | | nor | furaneol | | 3(2H)- | furanone, 4-hydroxy-5-methyl- | | 4- | hydroxy-5-methyl furan-3-one | | 4- | hydroxy-5-methyl furan-3(2H)-one | | 4- | hydroxy-5-methyl-2-hydrofuran-3-one | | 4- | hydroxy-5-methyl-2,3-dihydrofuran-3-one | | 4- | hydroxy-5-methyl-3-(2H)-furanone | | 4- | hydroxy-5-methyl-3-(2H)furanone | | 4- | hydroxy-5-methyl-3-furanone | | 4- | hydroxy-5-methyl-3(2H)-furanone | | 4- | hydroxy-5-methyl-3(2H)furanone | | 4- | hydroxy-5-methylfuran-3-one | | 4- | hydroxy-5-methylfuran-3(2H)-one | | 4- | hyroxy-5-methyl-3-furanone | | | methyl furaneol | | 5- | methyl-4-hydroxy-2,3-dihydrofuran-3-one | | 5- | methyl-4-hydroxy-3,2H-furanone | | 5- | methyl-4-hydroxy-3(2H)-furanone |
Articles:
| PubMed: | Comparative volatile profiles in soy sauce according to inoculated microorganisms. |
| PubMed: | An in vitro study reveals nutraceutical properties of Ananas comosus (L.) Merr. var. Mauritius fruit residue beneficial to diabetes. |
| PubMed: | Key aroma compounds in roasted in-shell peanuts. |
| PubMed: | Natural 4-hydroxy-2,5-dimethyl-3(2H)-furanone (Furaneol®). |
| PubMed: | The periodontopathogenic bacterium Eikenella corrodens produces an autoinducer-2-inactivating enzyme. |
| PubMed: | Structural basis for the enzymatic formation of the key strawberry flavor compound 4-hydroxy-2,5-dimethyl-3(2H)-furanone. |
| PubMed: | Studies on the key aroma compounds in raw (unheated) and heated Japanese soy sauce. |
| PubMed: | Effect of addition of commercial rosemary extracts on potent odorants in cooked beef. |
| PubMed: | D-Galacturonic acid as a highly reactive compound in nonenzymatic browning. 1. Formation of browning active degradation products. |
| PubMed: | An efficient method for the determination of furan derivatives in apple cider and wine by solid phase extraction and high performance liquid chromatography--diode array detector. |
| PubMed: | Volatile profiling of high quality hazelnuts (Corylus avellana L.): chemical indices of roasting. |
| PubMed: | DNA damage by genotoxic hydroxyhalofuranones: an in silico approach to MX. |
| PubMed: | Furanone derivatives from terrestrial Streptomyces spp. |
| PubMed: | Characterization of the key odorants in raw Italian hazelnuts ( Corylus avellana L. var. Tonda Romana) and roasted hazelnut paste by means of molecular sensory science. |
| PubMed: | Comparison of key aroma compounds in five different types of Japanese soy sauces by aroma extract dilution analysis (AEDA). |
| PubMed: | An efficient flow-photochemical synthesis of 5H-furanones leads to an understanding of torquoselectivity in cyclobutenone rearrangements. |
| PubMed: | Main odorants in Jura flor-sherry wines. Relative contributions of sotolon, abhexon, and theaspirane-derived compounds. |
| PubMed: | Evaluation of volatiles from two subtropical strawberry cultivars using GC-olfactometry, GC-MS odor activity values, and sensory analysis. |
| PubMed: | The volatile compounds in lamb fat are affected by the time of grazing. |
| PubMed: | Comparison of fermented soybean paste (Doenjang) prepared by different methods based on profiling of volatile compounds. |
| PubMed: | Convenient synthesis of 3,4-dichloro-5-hydroxy-2(5H)-furanone glycoconjugates. |
| PubMed: | Kinetics of the reactions of OH radicals with 2- and 3-methylfuran, 2,3- and 2,5-dimethylfuran, and E- and Z-3-hexene-2,5-dione, and products of OH + 2,5-dimethylfuran. |
| PubMed: | Maple syrup phytochemicals include lignans, coumarins, a stilbene, and other previously unreported antioxidant phenolic compounds. |
| PubMed: | Identification and formation of volatile components responsible for the characteristic aroma of mat rush (igusa). |
| PubMed: | Identification of novel aroma-active thiols in pan-roasted white sesame seeds. |
| PubMed: | Genome shuffling of Zygosaccharomyces rouxii to accelerate and enhance the flavour formation of soy sauce. |
| PubMed: | Stereochemical study of a novel tautomeric furanone, homofuraneol. |
| PubMed: | Development of a method based on on-line reversed phase liquid chromatography and gas chromatography coupled by means of an adsorption-desorption interface for the analysis of selected chiral volatile compounds in methyl jasmonate treated strawberries. |
| PubMed: | Identification of dihydromaltol (2,3-dihydro-5-hydroxy-6-methyl-4H-pyran-4-one) in Ryazhenka Kefir and comparative sensory impact assessment of related cycloenolones. |
| PubMed: | Improved derivatization technique for gas chromatography-mass spectrometry determination of 3-chloro-4-(dichloromethyl)-5-hydroxy-2(5H)-furanone in drinking water. |
| PubMed: | Cytotoxic and HIF-1alpha inhibitory compounds from Crossosoma bigelovii. |
| PubMed: | Decoding the key aroma compounds of a Hungarian-type salami by molecular sensory science approaches. |
| PubMed: | Absorption of 3(2H)-furanones by human intestinal epithelial Caco-2 cells. |
| PubMed: | Characterization of the key aroma compounds in pink guava (Psidium guajava L.) by means of aroma re-engineering experiments and omission tests. |
| PubMed: | Norfuraneol dephosphorylates eNOS at threonine 495 and enhances eNOS activity in human endothelial cells. |
| PubMed: | General, regiodefined access to alpha-substituted butenolides through metal-halogen exchange of 3-bromo-2-silyloxyfurans. Efficient synthesis of an anti-inflammatory gorgonian lipid. |
| PubMed: | Characterization of the most odor-active compounds in an American Bourbon whisky by application of the aroma extract dilution analysis. |
| PubMed: | Characterization of the aroma-active compounds in pink guava (Psidium guajava, L.) by application of the aroma extract dilution analysis. |
| PubMed: | Effects of water-soluble natural antioxidants on photosensitized oxidation of conjugated linoleic acid in an oil-in-water emulsion system. |
| PubMed: | A dual mechanism of 4-hydroxy-5-methyl-3[2H]-furanone inhibiting cellular melanogenesis. |
| PubMed: | Identification of characteristic aroma components of Thai fried chili paste. |
| PubMed: | The aroma side of the Maillard reaction. |
| PubMed: | Functional characterization of enone oxidoreductases from strawberry and tomato fruit. |
| PubMed: | Effects of the amino-carbonyl reaction of ribose and glycine on the formation of the 2(or 5)-ethyl-5(or 2)-methyl-4-hydroxy-3(2H)-furanone aroma component specific to miso by halo-tolerant yeast. |
| PubMed: | Characterization of the key aroma compounds in soy sauce using approaches of molecular sensory science. |
| PubMed: | Identification of sulfur volatiles in canned orange juices lacking orange flavor. |
| PubMed: | Effect of cysteine and cystine addition on sensory profile and potent odorants of extruded potato snacks. |
| PubMed: | Prooxidant action of furanone compounds: implication of reactive oxygen species in the metal-dependent strand breaks and the formation of 8-hydroxy-2'-deoxyguanosine in DNA. |
| PubMed: | The formation mechanism by yeast of 4-hydroxy-2(or 5)-ethyl-5(or 2)-methyl-3(2H)-furanone in Miso. |
| PubMed: | New constituents related to 3-methyl-2,4-nonanedione identified in green tea. |
| PubMed: | Two 2[5H]-furanones as possible signaling molecules in Lactobacillus helveticus. |
| PubMed: | FaQR, required for the biosynthesis of the strawberry flavor compound 4-hydroxy-2,5-dimethyl-3(2H)-furanone, encodes an enone oxidoreductase. |
| PubMed: | Functional effects of Japanese style fermented soy sauce (shoyu) and its components. |
| PubMed: | 4-Hydroxy-2,5-dimethyl-3(2H)-furanone (HDMF) production in simple media by lactic acid bacterium, Lactococcus lactis subsp. cremoris IFO 3427. |
| PubMed: | Characterization of dried whey protein concentrate and isolate flavor. |
| PubMed: | Altered expression of connexin43 in the inhibition of gap junctional intercellular communication by chlorohydroxyfuranones in WB-F344 rat liver epithelial cells. |
| PubMed: | Identification of potent odorants formed during the preparation of extruded potato snacks. |
| PubMed: | Odor-active constituents in fresh pineapple (Ananas comosus [L.] Merr.) by quantitative and sensory evaluation. |
| PubMed: | Alleviation of aflatoxin B1-induced oxidative stress in HepG2 cells by volatile extract from Allii Fistulosi Bulbus. |
| PubMed: | Concurrent phenomena contributing to the formation of the aroma of wine during aging in oak wood: an analytical study. |
| PubMed: | Identification of potent odorants in different cultivars of snake fruit [Salacca zalacca (Gaert.) Voss] using gas chromatography-olfactometry. |
| PubMed: | Quorum sensing in Clostridium difficile: analysis of a luxS-type signalling system. |
| PubMed: | Cytotoxicity of 3,4-dihalogenated 2(5H)-furanones. |
| PubMed: | Aroma extract dilution analysis of cv. Meeker (Rubus idaeus L.) red raspberries from Oregon and Washington. |
| PubMed: | Potent inhibition of gap junctional intercellular communication by 3-chloro-4-(dichloromethyl)-5-hydroxy-2(5H)-furanone (MX) in BALB/c 3T3 cells. |
| PubMed: | Bacterial and mammalian-cell genotoxicity of mixtures of chlorohydroxyfuranones, by-products of water chlorination. |
| PubMed: | Gas chromatography-olfactometry and chemical quantitative study of the aroma of six premium quality spanish aged red wines. |
| PubMed: | Evaluation of multiwavelength culture fluorescence for monitoring the aroma compound 4-hydroxy-2(or 5)-ethyl-5(or 2)-methyl-3(2H)-furanone (HEMF) production. |
| PubMed: | Effects of chlorohydroxyfuranones on 3-methylcholanthrene-induced neoplastic transformation in the two-stage transformation assay in C3H 10T1/2 cells. |
| PubMed: | Alternative pathway for the formation of 4,5-dihydroxy-2,3-pentanedione, the proposed precursor of 4-hydroxy-5-methyl-3(2H)-furanone as well as autoinducer-2, and its detection as natural constituent of tomato fruit. |
| PubMed: | Quantitative determination of sotolon, maltol and free furaneol in wine by solid-phase extraction and gas chromatography-ion-trap mass spectrometry. |
| PubMed: | Formation of 4-hydroxy-2,5-dimethyl-3[2H]-furanone by Zygosaccharomyces rouxii: identification of an intermediate. |
| PubMed: | Aroma extract dilution analysis of Cv. Marion (Rubus spp. hyb) and Cv. Evergreen (R. laciniatus L.) blackberries. |
| PubMed: | Sesquiterpenoids from Artemisia gilvescens and an anti-MRSA compound. |
| PubMed: | Formation of aroma compounds from ribose and cysteine during the Maillard reaction. |
| PubMed: | Potential of gas chromatography-orthogonal acceleration time-of-flight mass spectrometry (GC-oaTOFMS) in flavor research. |
| PubMed: | Investigation of the change in the flavor of a coffee drink during heat processing. |
| PubMed: | Maillard reaction products modulating the growth of human tumor cells in vitro. |
| PubMed: | Volatile constituents of glutathione--ribose model system and its antioxidant activity. |
| PubMed: | Formation of 5-methyl-4-hydroxy-3[2H]-furanone in cytosolic extracts obtained from Zygosaccharomyces rouxii. |
| PubMed: | Changes in the volatile compounds and in the chemical and physical properties of snake fruit (Salacca edulis Reinw) Cv. Pondoh during maturation. |
| PubMed: | Identification of potent odorants in Chinese jasmine green tea scented with flowers of Jasminum sambac. |
| PubMed: | Characterization of Trp(+) reversions in Escherichia coli strain WP2uvrA. |
| PubMed: | Formation of sulfur aroma compounds in reaction mixtures containing cysteine and three different forms of ribose. |
| PubMed: | Aroma biosynthesis in strawberry: s-adenosylmethionine:furaneol o-methyltransferase activity in ripening fruits. |
| PubMed: | Maillard reaction of D-glucose: identification of a colored product with conjugated pyrrole and furanone rings. |
| PubMed: | LuxS: its role in central metabolism and the in vitro synthesis of 4-hydroxy-5-methyl-3(2H)-furanone. |
| PubMed: | Volatile flavor components of stored nonfat dry milk. |
| PubMed: | Comparable DNA and chromosome damage in Chinese hamster ovary cells by chlorohydroxyfuranones. |
| PubMed: | Application of sec-butanol to the derivatization of hydroxyfuranones. |
| PubMed: | Stability of thiols in an aqueous process flavoring. |
| PubMed: | Halogenated 2,5-pyrrolidinediones: synthesis, bacterial mutagenicity in Ames tester strain TA-100 and semi-empirical molecular orbital calculations. |
| PubMed: | Identification of new heterocyclic nitrogen compounds from glucose-lysine and xylose-lysine maillard model systems. |
| PubMed: | Heterocyclic volatiles formed by heating cysteine or hydrogen sulfide with 4-hydroxy-5-methyl-3(2H)-furanone at pH 6.5. |
| PubMed: | Influence of pyrolytic and aqueous-phase reactions on the mechanism of formation of Maillard products. |
| PubMed: | A comparison of mutation spectra detected by the Escherichia coli lac(+) reversion assay and the Salmonella typhimurium his(+) reversion assay. |
| PubMed: | Inhibition of iron ion-induced oxidative damage of erythrocyte membranes and low density lipoprotein by a Maillard product, 4-hydroxy-2(or 5)-ethyl-5(or 2)-methyl-3(2H)-furanone (HEMF). |
| PubMed: | Mutation spectra of the drinking water mutagen 3-chloro-4-methyl-5-hydroxy-2(5H)-furanone (MCF) in Salmonella TA100 and TA104: comparison to MX. |
| PubMed: | Effect of food reductones on the generation of the pyrazine cation radical and on the formation of the mutagens in the reaction of glucose, glycine and creatinine. |
| PubMed: | Enzymatic synthesis of stable, odorless, and powdered furanone glucosides by sucrose phosphorylase. |
| PubMed: | Biomolecular-chemical screening: a novel screening approach for the discovery of biologically active secondary metabolites. III. New DNA-binding metabolites. |
| PubMed: | Reinvestigation of the reaction between 2-furancarboxaldehyde and 4-hydroxy-5-methyl-3(2H)-furanone. |
| PubMed: | Investigation of the reaction between 4-hydroxy-5-methyl-3(2H)-furanone and cysteine or hydrogen sulfide at pH 4.5. |
| PubMed: | Biosynthesis of 4-hydroxy-2,5-dimethyl-3(2H)-furanone and derivatives in in vitro grown strawberries. |
| PubMed: | Analysis of furanone, pyranone, and new heterocyclic colored compounds from sugar-glycine model Maillard systems. |
| PubMed: | Genotoxic activity of chlorohydroxyfuranones in the microscale micronucleus test on mouse lymphoma cells and the unscheduled DNA synthesis assay in rat hepatocytes. |
| PubMed: | The naturally occurring furanones: formation and function from pheromone to food. |
| PubMed: | Effect of media constituents on the formation by halophilic yeast of the 2 (or 5)-ethyl-5 (or 2)-methyl-4-hydroxy-3 (2H)-furanone aroma component specific to miso. |
| PubMed: | Promoting effects of 3-chloro-4-(dichloromethyl)-5-hydroxy-2(5H)-furanone on rat glandular stomach carcinogenesis initiated with N-methyl-N'-nitro-N-nitrosoguanidine. |
| PubMed: | Effects of protocatechuic acid, S-methylmethanethiosulfonate or 5-hydroxy-4-(2-phenyl-(E)ethenyl)-2(5H)-furanone(KYN-54) on 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone-induced pulmonary carcinogenesis in mice. |
| PubMed: | Synthesis of 4-hydroxy-3(2H)-furanone acyl derivatives and their anti-cataract effect on spontaneous cataract rats (ICR/f). |
| PubMed: | Antioxidative activities of 4-hydroxy-3(2H)-furanones and their anti-cataract effect on spontaneous cataract rat (ICR/f). |
| PubMed: | Absorption and induction of micronucleated peripheral reticulocytes in mice after oral administration of fragrant hydroxyfuranones generated in the Maillard reaction. |
| PubMed: | Identification of 2,5-dimethyl-4-hydroxy-3(2H)-furanone (DMHF) and 4-hydroxy-2(or 5)-ethyl-5(or 2)-methyl-3(2H)-furanone (HEMF) with DNA breaking activity in soy sauce. |
| PubMed: | Chemometric applications of thermally produced compounds as time-temperature integrators in aseptic processing of particulate foods. |
| PubMed: | Identification of adenine adducts formed in reaction of calf thymus DNA with mutagenic chlorohydroxyfuranones found in drinking water. |
| PubMed: | Bromine-, chlorine-, and mixed halogen-substituted 4-methyl-2(5H)-furanones: synthesis and mutagenic effects of halogen and hydroxyl group replacements. |
| PubMed: | Regressive effects of various chemopreventive agents on azoxymethane-induced aberrant crypt foci in the rat colon. |
| PubMed: | Detection of genotoxicity of polluted sea water using shellfish and the alkaline single-cell gel electrophoresis (SCE) assay: a preliminary study. |
| PubMed: | Inhibition of benzo[a]pyrene-induced mouse forestomach neoplasia and reduction of H2O2 concentration in human polymorphonuclear leucocytes by flavour components of Japanese-style fermented soy sauce. |
| PubMed: | Mucochloric acid action on phi X174 DNA: a comparison to other chlorine-substituted 2(5H)-furanones. |
| PubMed: | Enzymatic Kinetic Resolution of 5-Hydroxy-4-oxa-endo-tricyclo[5.2.1.0(2,6)]dec-8-en-3-ones: A Useful Approach to D-Ring Synthons for Strigol Analogues with Remarkable Stereoselectivity. |
| PubMed: | Synthesis of Highly Functionalized gamma-Butyrolactones from Activated Carbonyl Compounds and Dimethyl Acetylenedicarboxylate. |
| PubMed: | Identification of adducts formed in reaction of adenosine with 3-chloro-4-methyl-5-hydroxy-2(5H)-furanone, a bacterial mutagen present in chloride disinfected drinking water. |
| PubMed: | DNA breaking activity and mutagenicity of soy sauce: characterization of the active components and identification of 4-hydroxy-5-methyl-3(2H)-furanone. |
| PubMed: | DNA strand breaks induced through active oxygen radicals by fragrant component 4-hydroxy-2-hydroxymethyl-5-methyl-3(2H)-furanone in Maillard reaction of hexose/amino acid. |
| PubMed: | The determination of strong mutagen MX [3-chloro-4-(dichloromethyl)-5-hydroxy-2(5H)-furanone] in drinking water in China. |
| PubMed: | Male sex pheromone of cockroachEurycotis floridana (walker) (Blattidae, Polyzosteriinae): Role and composition of tergites 2 and 8 secretions. |
| PubMed: | Biodegradation of 4-methyl-5-nitrocatechol by Pseudomonas sp. strain DNT. |
| PubMed: | Inhibition of benzo[a]pyrene-induced mouse forestomach neoplasia by a principal flavor component of Japanese-style fermented soy sauce. |
| PubMed: | Structure-activity relationships of bacterial mutagens related to 3-chloro-4-(dichloromethyl)-5-hydroxy-2(5H)-furanone: an emphasis on the effect of stepwise removal of chlorine from the dichloromethyl group. |
| PubMed: | Salmonella typhimurium (TA100) mutagenicity of 3-chloro-4-(dichloromethyl)-5-hydroxy-2(5H)-furanone and its open- and closed-ring analogs. |
| PubMed: | Genotoxicity of drinking waters. |
| PubMed: | Ames mutagenicity and concentration of the strong mutagen 3-chloro-4-(dichloromethyl)-5-hydroxy-2(5H)-furanone and of its geometric isomer E-2-chloro-3-(dichloromethyl)-4-oxo-butenoic acid in chlorine-treated tap waters. |
|
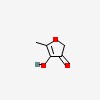 3D/inchi
3D/inchi














