Articles:
formic acid ethyl ester
Notes:
Fruit lifter. Found in various foods, e.g. cooked apple, orange juice, pineapple, other fruits, raw cabbage, coffee, black tea, wheat bread, white clover, sorghum. Used as a flavouring agent
| Flavor Demo Formulas | ||
| CAS Number: | 109-94-4 | 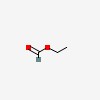 3D/inchi 3D/inchi
|
| ECHA EINECS - REACH Pre-Reg: | 203-721-0 | |
| FDA UNII: | 0K3E2L5553 | |
| Nikkaji Web: | J1.971B | |
| Beilstein Number: | 0906769 | |
| MDL: | MFCD00003294 | |
| CoE Number: | 339 | |
| XlogP3-AA: | 0.50 (est) | |
| Molecular Weight: | 74.07922000 | |
| Formula: | C3 H6 O2 | |
| BioActivity Summary: | listing | |
| NMR Predictor: | Predict (works with chrome, Edge or firefox) | |
Category: flavor and fragrance agents
US / EU / FDA / JECFA / FEMA / FLAVIS / Scholar / Patent Information:
| Google Scholar: | Search |
| Google Books: | Search |
| Google Scholar: with word "volatile" | Search |
| Google Scholar: with word "flavor" | Search |
| Google Scholar: with word "odor" | Search |
| Perfumer and Flavorist: | Search |
| Google Patents: | Search |
| US Patents: | Search |
| EU Patents: | Search |
| Pubchem Patents: | Search |
| PubMed: | Search |
| NCBI: | Search |
| FDA/DG SANTE Petitions, Reviews, Notices: | |
| 184.1295 | Ethyl formate View - review |
| JECFA Food Flavoring: | 26 ethyl formate |
| DG SANTE Food Flavourings: | 09.072 ethyl formate |
| FEMA Number: | 2434 ethyl formate |
| FDA: | No longer provide for the use of these seven synthetic flavoring substances |
| FDA Mainterm (SATF): | 109-94-4 ; ETHYL FORMATE |
| FDA Regulation: | |
| FDA PART 184 -- DIRECT FOOD SUBSTANCES AFFIRMED AS GENERALLY RECOGNIZED AS SAFE Subpart B--Listing of Specific Substances Affirmed as GRAS Sec. 184.1295 Ethyl formate. | |
Physical Properties:
Organoleptic Properties:
| Odor Type: ethereal | |
| Odor Strength: | high , recommend smelling in a 1.00 % solution or less |
| Substantivity: | 4 hour(s) at 100.00 % |
| ethereal green alcoholic rose cognac | |
| Odor Description: at 1.00 % in dipropylene glycol. | ethereal green alcohol rose cognac Luebke, William tgsc, (1989) |
| ethereal fermented winey cognac | |
| Odor Description: at 2.00 %. | Etherial, lifting, fermented, winey and cognac Mosciano, Gerard P&F 23, No. 3, 55, (1998) |
| Flavor Type: ethereal | |
| ethereal sweet grain fruity winey cognac | |
| Taste Description: at 200.00 ppm. | Etherial, sweet, grainy, fruity, winey and cognac Mosciano, Gerard P&F 23, No. 3, 55, (1998) |
| Odor and/or flavor descriptions from others (if found). | |
| Symrise | |
| Ethyl formate | |
| Odor Description: | strong, ethereal-fruity, like rum |
| Taste Description: | sweet, rum, fruity, baked fruit, whisky Useful in: mint, savory vegetable, fruity red, fruity yellow, fruity tropical, fruity others, sweet others, alcoholics. |
| Moellhausen | |
| ETHYL FORMATE | |
| Odor Description: | sweet, ethereal-fruity |
| Taste Description: | sweet, fruity |
| Prodasynth | |
| ETHYL FORMATE (> 98%) | |
| Odor Description: | FRUITY, RUM, STRONG, ETHEREAL |
| Taste Description: | SWEET,RUM,FRUITY,BAKED FRUIT,WHISKY |
Cosmetic Information:
| CosIng: | cosmetic data |
| Cosmetic Uses: |
perfuming agents |
Suppliers:
Safety Information:
| Preferred SDS: View | |
| European information : | |
| Most important hazard(s): | |
| Xn - Harmful. | |
|
R 11 - Highly flammable. R 20/22 - Harmful by inhalation and if swallowed. R 36/37 - Irritating to eyes and respiratory system. S 09 - Keep container in a well-ventilated place. S 16 - Keep away from sources of ignition - No Smoking. S 24 - Avoid contact with skin. S 26 - In case of contact with eyes, rinse immediately with plenty of water and seek medical advice. S 33 - Take precautionary measures against static discharge. | |
| Hazards identification | |
| Classification of the substance or mixture | |
| GHS Classification in accordance with 29 CFR 1910 (OSHA HCS) | |
| None found. | |
| GHS Label elements, including precautionary statements | |
| Pictogram | |
| Hazard statement(s) | |
| None found. | |
| Precautionary statement(s) | |
| None found. | |
| Human Experience: | |
| 4 % solution: no irritation or sensitization. | |
| Oral/Parenteral Toxicity: | |
|
oral-rat LD50 1850 mg/kg LUNGS, THORAX, OR RESPIRATION: DYSPNEA BEHAVIORAL: SOMNOLENCE (GENERAL DEPRESSED ACTIVITY) Food and Cosmetics Toxicology. Vol. 2, Pg. 327, 1964. oral-rabbit LD50 2075 mg/kg Industrial Medicine and Surgery. Vol. 41, Pg. 31, 1972. oral-guinea pig LD50 1110 mg/kg BEHAVIORAL: SOMNOLENCE (GENERAL DEPRESSED ACTIVITY) GASTROINTESTINAL: GASTRITIS Food and Cosmetics Toxicology. Vol. 2, Pg. 327, 1964. | |
| Dermal Toxicity: | |
|
skin-rabbit LD50 > 20 ml/kg AMA Archives of Industrial Hygiene and Occupational Medicine. Vol. 10, Pg. 61, 1954. subcutaneous-rabbit LDLo 1000 mg/kg Food and Cosmetics Toxicology. Vol. 16, Pg. 737, 1978. | |
| Inhalation Toxicity: | |
|
inhalation-rat LCLo 8000 ppm/4H AMA Archives of Industrial Hygiene and Occupational Medicine. Vol. 10, Pg. 61, 1954. | |
Safety in Use Information:
| Category: | flavor and fragrance agents | ||
| RIFM Fragrance Material Safety Assessment: Search | |||
| IFRA Code of Practice Notification of the 49th Amendment to the IFRA Code of Practice | |||
| Recommendation for ethyl formate usage levels up to: | |||
| 2.0000 % in the fragrance concentrate. | |||
| Use levels for FEMA GRAS flavoring substances on which the FEMA Expert Panel based its judgments that the substances are generally recognized as safe (GRAS). | |||
| The Expert Panel also publishes separate extensive reviews of scientific information on all FEMA GRAS flavoring substances and can be found at FEMA Flavor Ingredient Library | |||
| publication number: 3 | |||
| Click here to view publication 3 | |||
| average usual ppm | average maximum ppm | ||
| baked goods: | - | 98.00000 | |
| beverages(nonalcoholic): | - | 9.40000 | |
| beverages(alcoholic): | - | 10.00000 | |
| breakfast cereal: | - | - | |
| cheese: | - | - | |
| chewing gum: | - | 430.00000 | |
| condiments / relishes: | - | - | |
| confectionery froastings: | - | - | |
| egg products: | - | - | |
| fats / oils: | - | - | |
| fish products: | - | - | |
| frozen dairy: | - | 21.00000 | |
| fruit ices: | - | 21.00000 | |
| gelatins / puddings: | 0.35000 | 11.00000 | |
| granulated sugar: | - | - | |
| gravies: | - | - | |
| hard candy: | - | 50.00000 | |
| imitation dairy: | - | - | |
| instant coffee / tea: | - | - | |
| jams / jellies: | - | - | |
| meat products: | - | - | |
| milk products: | - | - | |
| nut products: | - | - | |
| other grains: | - | - | |
| poultry: | - | - | |
| processed fruits: | - | - | |
| processed vegetables: | - | - | |
| reconstituted vegetables: | - | - | |
| seasonings / flavors: | - | - | |
| snack foods: | - | - | |
| soft candy: | - | - | |
| soups: | - | - | |
| sugar substitutes: | - | - | |
| sweet sauces: | - | - | |
Safety References:
| European Food Safety Authority (EFSA) reference(s): | |
| Flavouring Group Evaluation 2, Revision 1 (FGE.02) : Branched- and straight-chain aliphatic saturated primary alcohols and related esters of primary alcohols and straight-chain carboxylic acids and one straight-chain aldehyde from chemical groups 1 and 2 (Commission Regulation (EC) No 1565/2000 of 18 July 2000) - Opinion of the Scientific Panel on Food Additives, Flavourings, Processing Aids and Materials in contact with Food (AFC) View page or View pdf | |
| Scientific Opinion on the safety and efficacy of straight-chain primary aliphatic alcohols/aldehydes/acids, acetals and esters with esters containing saturated alcohols and acetals containing saturated aldehydes (chemical group 1) when used as flavourings for all animal species View page or View pdf | |
| EPI System: | View |
| NIOSH International Chemical Safety Cards: | search |
| NIOSH Pocket Guide: | search |
| AIDS Citations: | Search |
| Cancer Citations: | Search |
| Toxicology Citations: | Search |
| EPA Substance Registry Services (TSCA): | 109-94-4 |
| EPA ACToR: | Toxicology Data |
| EPA Substance Registry Services (SRS): | Registry |
| Laboratory Chemical Safety Summary : | 8025 |
| National Institute of Allergy and Infectious Diseases: | Data |
| WISER: | UN 1190 |
| WGK Germany: | 1 |
| ethyl formate | |
| Chemidplus: | 0000109944 |
| EPA/NOAA CAMEO: | hazardous materials |
| RTECS: | 109-94-4 |
References:
| ethyl formate | |
| NIST Chemistry WebBook: | Search Inchi |
| Canada Domestic Sub. List: | 109-94-4 |
| Pubchem (cid): | 8025 |
| Pubchem (sid): | 134974012 |
| Flavornet: | 109-94-4 |
| Pherobase: | View |
Other Information:
| (IUPAC): | Atomic Weights of the Elements 2011 (pdf) |
| Videos: | The Periodic Table of Videos |
| tgsc: | Atomic Weights use for this web site |
| (IUPAC): | Periodic Table of the Elements |
| FDA Substances Added to Food (formerly EAFUS): | View |
| CHEBI: | View |
| CHEMBL: | View |
| HMDB (The Human Metabolome Database): | HMDB31229 |
| FooDB: | FDB003253 |
| YMDB (Yeast Metabolome Database): | YMDB01684 |
| Export Tariff Code: | 2915.13.5000 |
| Typical G.C. | |
| VCF-Online: | VCF Volatile Compounds in Food |
| ChemSpider: | View |
| Formulations/Preparations: •areginal...is mixture of methyl formate and ethyl formate. •assay: 95% minimum. •grades: technical; fcc. | |
Potential Blenders and core components note
Potential Uses:
| almond | FR | |
| apple | FR | |
| apricot | FR | |
| beer | FL | |
| blackberry | FR | |
| brandy | FL | |
| butter | FR | |
| clary sage oil replacer | FR | |
| coffee | FR | |
| currant | FR | |
| ethereal | ||
| fruit | FR | |
| grape | FR | |
| honey | FR | |
| lemon | FR | |
| malt | FR | |
| melon | FR | |
| mulberry | FR | |
| peach | FR | |
| pear | FR | |
| pineapple | FR | |
| plum mirabelle plum | FR | |
| quince | FR | |
| raspberry | FR | |
| reseda | FR | |
| saffron | FR | |
| strawberry | FR | |
| tea | FL | |
| topnotes | ||
| valerian | FL/FR | |
| whiskey | FL |
Occurrence (nature, food, other): note
| apple fruit Search Trop Picture | |
| beer Search PMC Picture | |
| brandy Search PMC Picture | |
| butter Search PMC Picture | |
| cabbage Search Trop Picture | |
| jasmin absolute china @ trace% Data GC Search Trop Picture | |
| onion Search Trop Picture | |
| pineapple fruit Search Trop Picture | |
| plum fruit Search Trop Picture | |
| quince fruit Search Trop Picture | |
| raspberry red raspberry fruit Search Trop Picture | |
| rum Search PMC Picture | |
| sage clary sage Search Trop Picture | |
| strawberry wild strawberry fruit Search Trop Picture | |
| vinegar Search Picture |
Synonyms:
| areginal | |
| carboxylic acid oxaethane | |
| ethyl ester of formic acid | |
| nat. | ethyl formate |
| ethyl formate (natural) | |
| ethyl formate FCC | |
| ethyl formate natural | |
| ethyl formate naturel | |
| ethyl formic ester | |
| ethyl methanoate | |
| formic acid ethyl ester | |
| formic ether |




























