|
Category: cosmetic, flavor and fragrance agents
US / EU / FDA / JECFA / FEMA / FLAVIS / Scholar / Patent Information:
Physical Properties:
| Appearance: | white to yellowish orange solid (est) |
| Assay: | 99.00 to 100.00 %
|
| Food Chemicals Codex Listed: | No |
| Melting Point: | 45.00 to 47.00 °C. @ 760.00 mm Hg
|
| Boiling Point: | 201.00 to 203.00 °C. @ 760.00 mm Hg
|
| Vapor Pressure: | 0.221000 mmHg @ 25.00 °C. (est) |
| Flash Point: | 173.00 °F. TCC ( 78.33 °C. )
|
| logP (o/w): | 2.360 |
| Soluble in: |
| | alcohol | | | water, 3615 mg/L @ 25 °C (est) | | | water, 6050 mg/L @ 25 °C (exp) |
| Insoluble in: |
| | water |
Organoleptic Properties:
| |
| Odor Type: medicinal |
| |
| | sweet medicinal phenolic rooty coffee |
Odor Description:
at 0.10 % in propylene glycol. | sweet medicinal phenolic rooty coffee |
| |
| |
| Flavor Type: burnt |
| |
| | sweet burnt smoky |
Taste Description:
| sweet burnt smoky |
| |
| Odor and/or flavor descriptions from others (if found). |
| |
| |
Cosmetic Information:
Suppliers:
| Berjé |
| 2,6-Xylenol
|
| Media |
| EMD Millipore |
| For experimental / research use only. |
| 2,6-Dimethylphenol
|
| Glentham Life Sciences |
| 2,6-Dimethylphenol
|
| Lluch Essence |
| 2,6-XYLENOL
|
| M&U International |
| 2,6-Dimethyl Phenol
|
| Penta International |
| 2,6-XYLENOL
|
| Sigma-Aldrich |
| 2,6-Xylenol, ≥99%
Odor: medicinal |
| Certified Food Grade Products |
| Synerzine |
| 2,6-Xylenol
|
| TCI AMERICA |
| For experimental / research use only. |
| 2,6-Dimethylphenol >99.0%(GC)
|
| Vigon International |
| Xylenol-2 6
Odor: SWEET, TARRY-LIKE |
Safety Information:
| Preferred SDS: View |
| European information : |
| Most important hazard(s): | | T N - Toxic, Dangerous for the environment. |
R 24/25 - Toxic in contact with skin and if swallowed.
R 34 - Causes burns.
R 51/53 - Toxic to aquatic organisms, may cause long-term adverse effects in the aquatic environment.
S 01/02 - Keep locked up and out of the reach of children.
S 20/21 - When using do not eat, drink or smoke.
S 24/25 - Avoid contact with skin and eyes.
S 26 - In case of contact with eyes, rinse immediately with plenty of water and seek medical advice.
S 36/37/39 - Wear suitable clothing, gloves and eye/face protection.
S 45 - In case of accident or if you feel unwell seek medical advice immediately.
S 61 - Avoid release to the environment. Refer to special instructions/safety data sheet.
|
| |
| Hazards identification |
| |
| Classification of the substance or mixture |
| GHS Classification in accordance with 29 CFR 1910 (OSHA HCS) |
| None found. |
| GHS Label elements, including precautionary statements |
| |
| Pictogram | |
| |
| Hazard statement(s) |
| None found. |
| Precautionary statement(s) |
| None found. |
| Oral/Parenteral Toxicity: |
oral-rat LD50 296 mg/kg
(Maazik, 1968)
oral-rabbit LD50 700 mg/kg
(Maazik, 1968)
oral-mouse LD50 450 mg/kg
(Larionov, 1976)
intraperitoneal-mouse LD50 150 mg/kg
National Technical Information Service. Vol. AD691-490
oral-rat LD50 406 mg/kg
(Larionov, 1976)
oral-mouse LD50 479 mg/kg
(Maazik, 1968)
intravenous-mouse LD50 80 mg/kg
BEHAVIORAL: GENERAL ANESTHETIC
BEHAVIORAL: SLEEP
Journal of Medicinal Chemistry. Vol. 23, Pg. 1350, 1980.
oral-mouse LD50 450 mg/kg
BEHAVIORAL: SOMNOLENCE (GENERAL DEPRESSED ACTIVITY)
BEHAVIORAL: CONVULSIONS OR EFFECT ON SEIZURE THRESHOLD
LUNGS, THORAX, OR RESPIRATION: OTHER CHANGES
Gigiena Truda i Professional'nye Zabolevaniya. Labor Hygiene and Occupational Diseases. Vol. 20(4), Pg. 43, 1976.
oral-rabbit LD50 700 mg/kg
BEHAVIORAL: MUSCLE CONTRACTION OR SPASTICITY)
LUNGS, THORAX, OR RESPIRATION: DYSPNEA
BEHAVIORAL: ATAXIA
Hygiene and Sanitation Vol. 33(7-9), Pg. 329, 1968.
oral-rat LD50 296 mg/kg
Gigiena Truda i Professional'naya Patologiya v Estonskoi SSR. Labor Hygiene and Occupational Pathology in the Estonian SSR. Vol. 8, Pg. 145, 1972.
|
| Dermal Toxicity: |
skin-rabbit LD50 1000 mg/kg
Industrial Hygiene Foundation of America, Chemical and Toxicological Series, Bulletin. Vol. 6, Pg. 1, 1967.
skin-rat LD50 2325 mg/kg
BEHAVIORAL: CONVULSIONS OR EFFECT ON SEIZURE THRESHOLD
BEHAVIORAL: SOMNOLENCE (GENERAL DEPRESSED ACTIVITY)
LUNGS, THORAX, OR RESPIRATION: OTHER CHANGES
Gigiena Truda i Professional'nye Zabolevaniya. Labor Hygiene and Occupational Diseases. Vol. 20(4), Pg. 43, 1976.
skin-mouse LD50 920 mg/kg
Gigiena Truda i Professional'nye Zabolevaniya. Labor Hygiene and Occupational Diseases. Vol. 18(2), Pg. 58, 1974.
|
| Inhalation Toxicity: |
inhalation-mammal (species unspecified) LC > 270 mg/m3
Gigiena Truda i Professional'nye Zabolevaniya. Labor Hygiene and Occupational Diseases. Vol. 20(4), Pg. 43, 1976.
|
Safety in Use Information:
| Category: | cosmetic, flavor and fragrance agents |
| RIFM Fragrance Material Safety Assessment: Search |
| IFRA Code of Practice Notification of the 49th Amendment to the IFRA Code of Practice |
| Recommendation for 2,6-xylenol usage levels up to: | | | 0.0500 % in the fragrance concentrate.
|
| |
| Maximised Survey-derived Daily Intakes (MSDI-EU): | 1.70 (μg/capita/day) |
| Maximised Survey-derived Daily Intakes (MSDI-USA): | 1.00 (μg/capita/day) |
| Use levels for FEMA GRAS flavoring substances on which the FEMA Expert Panel based its judgments that the substances are generally recognized as safe (GRAS). |
| The Expert Panel also publishes separate extensive reviews of scientific information on all FEMA GRAS flavoring substances and can be found at FEMA Flavor Ingredient Library |
| publication number: 4 |
| Click here to view publication 4 |
| | average usual ppm | average maximum ppm |
| baked goods: | - | - |
| beverages(nonalcoholic): | - | 1.00000 |
| beverages(alcoholic): | - | - |
| breakfast cereal: | - | - |
| cheese: | - | - |
| chewing gum: | - | - |
| condiments / relishes: | - | 1.00000 |
| confectionery froastings: | - | - |
| egg products: | - | - |
| fats / oils: | - | - |
| fish products: | - | - |
| frozen dairy: | - | - |
| fruit ices: | - | - |
| gelatins / puddings: | - | - |
| granulated sugar: | - | - |
| gravies: | - | - |
| hard candy: | - | - |
| imitation dairy: | - | - |
| instant coffee / tea: | - | - |
| jams / jellies: | - | - |
| meat products: | - | - |
| milk products: | - | - |
| nut products: | - | - |
| other grains: | - | - |
| poultry: | - | - |
| processed fruits: | - | - |
| processed vegetables: | - | - |
| reconstituted vegetables: | - | - |
| seasonings / flavors: | - | - |
| snack foods: | - | - |
| soft candy: | - | - |
| soups: | - | - |
| sugar substitutes: | - | - |
| sweet sauces: | - | - |
Safety References:
| European Food Safety Athority(EFSA): | Flavor usage levels; Subacute, Subchronic, Chronic and Carcinogenicity Studies; Developmental / Reproductive Toxicity Studies; Genotoxicity Studies... |
| European Food Safety Authority (EFSA) reference(s): |
Opinion of the Scientific Panel on food additives, flavourings, processing aids and materials in contact with food (AFC) related to Flavouring Group Evaluation 22 (FGE.22): Ring-substituted phenolic substances from chemical groups 21 and 25 (Commission Regulation (EC) No 1565/2000 of 18 July 2000)
View page or View pdf |
Flavouring Group Evaluation 58 (FGE.58) Consideration of phenol derivatives evaluated by JECFA (55th meeting) structurally related to ring substituted phenolic substances evaluated by EFSA in FGE.22 (2006) (Commission Regulation (EC) No 1565/2000 of 18 July 2000) - Opinion of the Scientific Panel on Food Additives, Flavourings, Processing Aids and Materials in contact with Food (AFC)
View page or View pdf |
Flavouring Group Evaluation 60 (FGE.60): Consideration of eugenol and related hydroxyallylbenzene derivatives evaluated by JECFA (65th meeting) structurally related to ring- substituted phenolic substances evaluated by EFSA in FGE.22 (2006)
View page or View pdf |
Scientific Opinion on Flavouring Group Evaluation 22, Revision 1 (FGE.22Rev1): Ring-substituted phenolic substances from chemical groups 21 and 25
View page or View pdf |
Scientific Opinion on the safety and efficacy of phenol derivatives containing ring-alkyl, ring-alkoxy and side-chains with an oxygenated functional group (chemical group 25) when used as flavourings for all species
View page or View pdf |
| EPI System: | View |
| EPA-Iris: | IRIS |
| AIDS Citations: | Search |
| Cancer Citations: | Search |
| Toxicology Citations: | Search |
| EPA GENetic TOXicology: | Search |
| EPA Substance Registry Services (TSCA): | 576-26-1 |
| EPA ACToR: | Toxicology Data |
| EPA Substance Registry Services (SRS): | Registry |
| Laboratory Chemical Safety Summary : | 11335 |
| National Institute of Allergy and Infectious Diseases: | Data |
| WISER: | UN 2261 |
| WGK Germany: | 2 |
| | 2,6-dimethylphenol |
| Chemidplus: | 0000576261 |
| EPA/NOAA CAMEO: | hazardous materials |
| RTECS: | ZE6125000 for cas# 576-26-1 |
References:
Other Information:
| (IUPAC): | Atomic Weights of the Elements 2011 (pdf) |
| Videos: | The Periodic Table of Videos |
| tgsc: | Atomic Weights use for this web site |
| (IUPAC): | Periodic Table of the Elements |
| FDA Substances Added to Food (formerly EAFUS): | View |
| FDA Indirect Additives used in Food Contact Substances: | View |
| CHEBI: | View |
| CHEMBL: | View |
| HMDB (The Human Metabolome Database): | HMDB32150 |
| FooDB: | FDB008878 |
| Export Tariff Code: | 2907.19.8000 |
| Typical G.C. |
| VCF-Online: | VCF Volatile Compounds in Food |
| ChemSpider: | View |
| Wikipedia: | View |
Formulations/Preparations:
•pitt-consol xylenol 235 is a synthetic 2,6-xylenol. homolog distribution, wt%: ortho cresol, 0.5; 2,6-xylenols, 92; 2,42,5 xylenols, 0.5; metapara cresol, 7.
•grade or purity: 99%
•pitt-consol xylenol 410 is a synthetic xylenol blend homolog distribution, wt %: 2,6-xylenol, 1; 2,4-xylenol, 42; 2,5-xylenol, 43; 2,3-xylenol group, 14. xylenol 410
|
Potential Blenders and core components note
| |
| For Odor |
| alliaceous |
| | methyl furfuryl disulfide | FL/FR |
| balsamic |
| 2- | acetyl furan | FL/FR |
| burnt |
| | rum ether | FL/FR |
| | coffee dione | FL/FR |
| | cyclotene | FL/FR |
| chocolate |
| 2,6- | dimethyl pyrazine | FL/FR |
| 2,4,5- | trimethyl thiazole | FL/FR |
| cocoa |
| 2- | methyl butyraldehyde | FL/FR |
| coffee |
| | coffee difuran | FL/FR |
| | furfuryl mercaptan | FL/FR |
| 1- | hydroxy-2-butanone | FL/FR |
| 2- | methyl-3-,5 or 6-(furfuryl thio) pyrazine | FL/FR |
| dirty |
| 3,3- | dimethyl-5- and 6-isopropyl bicyclo(2.2.2)-5,7-octadiene-2-one | |
| 2- | ethyl fenchol | FL/FR |
| | octyl phenyl acetate | FL/FR |
| | rhubarb pyran | FR |
| | tropical thiazole | FL/FR |
| green |
| | galbanum oil | FL/FR |
| | valerian rhizome oil CO2 extract china | FL/FR |
| herbal |
| | ajowan seed oil | FL/FR |
| 1- | allyl-2,2,7,7-tetramethyl cycloheptanol | FR |
| | barosma betulina leaf oil | FL/FR |
| 2,10- | epoxypinane | FR |
| | matricaria chamomilla flower oil | FL/FR |
| white | thyme oil | FL/FR |
| | thyme oil (thymus zygis gracillis) spain | FL/FR |
| red | thyme oil spain | FL/FR |
| | thymol | FL/FR |
| | valerian rhizome oil | FL/FR |
| | valerian rhizome oil china | FL/FR |
| leathery |
| 2-tert- | butyl-6-methyl phenol | FR |
| musty |
| | menthofuran | FL/FR |
| nutty |
| 3,5- | cocoa pyrazine | FL/FR |
| 2,3- | dimethyl pyrazine | FL/FR |
| 4,5- | dimethyl-2-ethyl-3-thiazoline | FL/FR |
| | filbert heptenone | FL/FR |
| 5- | methyl quinoxaline | FL/FR |
| 2- | methyl thio-3,5 or 6-methyl pyrazine | FL/FR |
| 2,3,5,6- | tetramethyl pyrazine | FL/FR |
| | vinyl sulfurol | FL/FR |
| ortho- | cresol | FR |
| meta- | cresol | FR |
| 4- | methyl-2,6-dimethoxyphenol | FL/FR |
| 2- | propyl phenol | FL/FR |
| 4- | propyl phenol | FL/FR |
| 4- | vinyl phenol | FL/FR |
| popcorn |
| 2- | acetyl pyrazine | FL/FR |
| smoky |
| | pyroligneous acids | FL/FR |
| spicy |
| 4- | methyl guaiacol | FL/FR |
| sulfurous |
| | benzothiazole | FL/FR |
| | cassis pentanone | FL/FR |
| | furfuryl thioacetate | FL/FR |
| S- | furfuryl thioformate | FL/FR |
| 2- | methyl 5-(methyl thio) furan | FL/FR |
| tonka |
| | melilot absolute | FR |
| vegetable |
| 1- | furfuryl pyrrole | FL/FR |
| | vetiver oil haiti | FL/FR |
| | vetiver resinoid | FR |
| | vetiverol | FL/FR |
| | vetiveryl acetate | FL/FR |
| |
| For Flavor |
| |
| No flavor group found for these |
| | chavicol | FL |
| 2,5- | dimethyl-3-thiofuroyl furan | FL |
| 3,3- | dimethyl-5- and 6-isopropyl bicyclo(2.2.2)-5,7-octadiene-2-one | |
| | elecampane root absolute | FL |
| | elecampane root oil | FL |
| | melilot oleoresin | FL |
| 2- | methyl-3-,5 or 6-(furfuryl thio) pyrazine | FL/FR |
| | octyl phenyl acetate | FL/FR |
| 4- | propyl phenol | FL/FR |
| 2- | propyl phenol | FL/FR |
| | vetiveryl acetate | FL/FR |
| alliaceous |
| | benzyl mercaptan | FL |
| | dicyclohexyl disulfide | FL |
| | tropical thiazole | FL/FR |
| brown |
| 1- | hydroxy-2-butanone | FL/FR |
| burnt |
| | furfuryl alcohol | FL |
| 2- | methyl quinoxaline | FL |
| | rum ether | FL/FR |
| caramellic |
| | caramel furanone | FL |
| | cyclotene | FL/FR |
| coffee |
| | coffee difuran | FL/FR |
| | coffee dione | FL/FR |
| | coffee pyrazine | FL |
| | difurfuryl ether | FL |
| | diisoamyl thiomalate | FL |
| 2,4- | dimethyl thiazole | FL |
| | furfuryl mercaptan | FL/FR |
| | methyl furfuryl disulfide | FL/FR |
| 2- | thiophene thiol | FL |
| earthy |
| | difurfuryl sulfide | FL |
| 2- | ethyl fenchol | FL/FR |
| fruity |
| | furfuryl propionate | FL |
| | valerian rhizome oil | FL/FR |
| | valerian rhizome oil china | FL/FR |
| | valerian rhizome oil CO2 extract china | FL/FR |
| fusel |
| 2- | methyl butyraldehyde | FL/FR |
| green |
| | cassis pentanone | FL/FR |
| | galbanum oil | FL/FR |
| 2- | methyl-5-isopropyl pyrazine | FL |
| herbal |
| | ajowan seed oil | FL/FR |
| | barosma betulina leaf oil | FL/FR |
| | matricaria chamomilla flower oil | FL/FR |
| white | thyme oil | FL/FR |
| | thyme oil (thymus zygis gracillis) spain | FL/FR |
| red | thyme oil spain | FL/FR |
| meaty |
| | benzothiazole | FL/FR |
| 2,6- | dimethyl pyrazine | FL/FR |
| 2- | methyl 3-(methyl thio) furan | FL |
| mustard |
| | furfuryl methyl ether | FL |
| musty |
| | menthofuran | FL/FR |
| 2- | methyl 5-(methyl thio) furan | FL/FR |
| nutty |
| 2- | acetyl furan | FL/FR |
| 3,5(6)- | cocoa pyrazine | FL |
| 3,5- | cocoa pyrazine | FL/FR |
| 2,3- | dimethyl pyrazine | FL/FR |
| 4,5- | dimethyl-2-ethyl-3-thiazoline | FL/FR |
| | filbert heptenone | FL/FR |
| 5- | methyl quinoxaline | FL/FR |
| 2- | methyl thio-3,5 or 6-methyl pyrazine | FL/FR |
| 2,3,5,6- | tetramethyl pyrazine | FL/FR |
| 2,4,5- | trimethyl thiazole | FL/FR |
| | vinyl sulfurol | FL/FR |
| onion |
| | furfuryl isopropyl sulfide | FL |
| phenolic |
| 4- | methyl-2,6-dimethoxyphenol | FL/FR |
| | thymol | FL/FR |
| 4- | vinyl phenol | FL/FR |
| roasted |
| 2- | acetyl pyrazine | FL/FR |
| | ethyl 3-(furfuryl thio) propionate | FL |
| | furfuryl thioacetate | FL/FR |
| smoky |
| | pyroligneous acids | FL/FR |
| spicy |
| 4- | methyl guaiacol | FL/FR |
| winter | savory oil | FL |
| sulfurous |
| | butyl mercaptan | FL |
| | ethyl methyl sulfide | FL |
| S- | ethyl thioacetate | FL |
| S- | furfuryl thioformate | FL/FR |
| | furfuryl thiopropionate | FL |
| 2- | thienyl mercaptan | FL |
| vegetable |
| 1- | furfuryl pyrrole | FL/FR |
| | radish isothiocyanate | FL |
| woody |
| | vetiver oil haiti | FL/FR |
| | vetiverol | FL/FR |
| |
Potential Uses:
Occurrence (nature, food, other): noteSynonyms:
| 2,6- | dimethyl phenol | | 2,6- | dimethyl-hydroxy-benzene | | 2,6- | dimethyl-phenol | | 2,6- | dimethylphenol | | 2- | hydroxy-1,3-dimethyl benzene | | 1- | hydroxy-2,6-dimethyl benzene | | 2- | hydroxy-m-xylene | | | phenol, 2,6-dimethyl- | | | xylenol-2 6 |
Articles:
| US Patents: | 3,946,080 - Flavouring and perfuming ingredients |
| PubMed: | Cyclometalated N-Heterocyclic Carbene Complexes of Ruthenium for Access to Electron-Rich Silylene Complexes That Bind the Lewis Acids CuOTf and AgOTf. |
| US Patents: | 3,952,024 - Furfurylthioacetone |
| PubMed: | Identification of oxidative coupling products of xylenols arising from laboratory-scale phytoremediation. |
| PubMed: | Metal-organic framework UiO-66 coated stainless steel fiber for solid-phase microextraction of phenols in water samples. |
| PubMed: | Engineering the expression and characterization of two novel laccase isoenzymes from Coprinus comatus in Pichia pastoris by fusing an additional ten amino acids tag at N-terminus. |
| PubMed: | Characterisation of volatile compounds in a smoke flavouring from rice husk. |
| PubMed: | Characterization of a bifunctional O- and N-glucosyltransferase from Vitis vinifera in glucosylating phenolic compounds and 3,4-dichloroaniline in Pichia pastoris and Arabidopsis thaliana. |
| PubMed: | Aluminum complexes incorporating symmetrical and asymmetrical tridentate pincer type pyrrolyl ligands: synthesis, characterization and reactivity study. |
| PubMed: | Silane-isocyanide coupling involving 1,1-insertion of XylNC into the Si-H bond of a σ-silane ligand. |
| PubMed: | Synthesis, structural characterization and catalytic evaluation of the ring-opening polymerization of discrete five-coordinate alkyl aluminium complexes. |
| PubMed: | A computational study on enzymatically driven oxidative coupling of chlorophenols: an indirect dehalogenation reaction. |
| PubMed: | Measurement of detergent concentration using 2,6-dimethylphenol in membrane-protein crystallization. |
| PubMed: | Heterologous expression and characterization of a novel laccase isoenzyme with dyes decolorization potential from Coprinus comatus. |
| PubMed: | 4-Chloropropofol enhances chloride currents in human hyperekplexic and artificial mutated glycine receptors. |
| PubMed: | Oxidation of 2,6-dimethylaniline by the Fenton, electro-Fenton and photoelectro-Fenton processes. |
| PubMed: | Montmorillonite K-10 clay-catalyzed Ferrier rearrangement of 2-C-hydroxymethyl-d-glycals, 3,4,6-tri-O-alkyl-d-glycals, and 3,4-(dihydro-2H-pyran-5-yl)methanol: a few unexpected domino transformations. |
| PubMed: | Anion exchange induced tunable catalysis properties of an uncommon butterfly-like tetranuclear copper(II) cluster and magnetic characterization. |
| PubMed: | The anticonvulsant effects of propofol and a propofol analog, 2,6-diisopropyl-4-(1-hydroxy-2,2,2-trifluoroethyl)phenol, in a 6 Hz partial seizure model. |
| PubMed: | Gas-phase phenol methylation over Mg/Me/O (Me = Al, Cr, Fe) catalysts: mechanistic implications due to different acid-base and dehydrogenating properties. |
| PubMed: | Cyanation of arenes via iridium-catalyzed borylation. |
| PubMed: | Identification of 4-[4-(4-fluoro-phenyl)-thiazol-2-ylamino]-2,6-dimethyl-phenol (KR-33749) as an inhibitor of 5-lipoxygenase with potent antiinflammatory activity. |
| PubMed: | Oligochlorophens are potent inhibitors of Bacillus anthracis. |
| PubMed: | Mitochondrial biotransformation of omega-(phenoxy)alkanoic acids, 3-(phenoxy)acrylic acids, and omega-(1-methyl-1H-imidazol-2-ylthio)alkanoic acids: a prodrug strategy for targeting cytoprotective antioxidants to mitochondria. |
| PubMed: | Chemical oxidation of 2,6-dimethylaniline in the fenton process. |
| PubMed: | Chemical oxidation of 2,6-dimethylaniline by electrochemically generated Fenton's reagent. |
| PubMed: | Comparative study on determination of antioxidant and membrane activities of propofol and its related compounds. |
| PubMed: | Reaction of an oxaruthenacycle with DMAD. Stoichiometric transformations of 2,6-xylenol to allylic phenols and benzopyrans via sp(3) C-H bond cleavage reaction. |
| PubMed: | Coupling poly-(methacrylic acid-co-ethylene glycol dimethacrylate) monolith microextraction to capillary electrophoresis for the determination of phenols in water samples. |
| PubMed: | Novel multiwalled carbon nanotubes-polyaniline composite film coated platinum wire for headspace solid-phase microextraction and gas chromatographic determination of phenolic compounds. |
| PubMed: | Two methods for catalytic generation of reactive enolates promoted by a chiral poly gd complex: application to catalytic enantioselective protonation reactions. |
| PubMed: | Carbonic anhydrase inhibitors. Inhibition of human erythrocyte isozymes I and II with a series of antioxidant phenols. |
| PubMed: | Proton-coupled electron-transfer oxidation of phenols by hexachloroiridate(IV). |
| PubMed: | Retention time prediction of compounds in Grob standard mixture for apolar capillary columns in temperature-programmed gas chromatography. |
| PubMed: | Selective and stability-indicating methods for the simultaneous determination of mexiletine hydrochloride and/or its related substance: 2,6-dimethylphenol. |
| PubMed: | [New promising antioxidants based on 2,6-dimethylphenol]. |
| PubMed: | Photodegradation of bisphenol A and related compounds under natural-like conditions in the presence of riboflavin: kinetics, mechanism and photoproducts. |
| PubMed: | Synthesis of (1-adamantylimido)vanadium(V) complexes containing aryloxo, ketimide ligands: effect of ligand substituents in olefin insertion/metathesis polymerization. |
| PubMed: | [New efficient producers of fungal laccases]. |
| PubMed: | Versatile scorpionates and new developments in the denticity changes of NNCp hybrid scorpionate/cyclopentadienyl ligands in Sc and Y compounds: from kappa1-Neta5-Cp to kappa2-NNeta5-Cp. |
| PubMed: | A catalytic enantioselective conjugate addition of cyanide to enones. |
| PubMed: | Selective benzylic C-C coupling catalyzed by a bioinspired dicopper complex. |
| PubMed: | Synthesis of dimeric phenol derivatives and determination of in vitro antioxidant and radical scavenging activities. |
| PubMed: | Synthesis, magnetic behaviour, and X-ray structures of dinuclear copper complexes with multiple bridges. Efficient and selective catalysts for polymerization of 2,6-dimethylphenol. |
| PubMed: | Detailed examination of the degradation of phenol derivatives under oxygen delignification conditions. |
| PubMed: | Determination of chlorine dioxide in water by gas chromatography-mass spectrometry. |
| PubMed: | Laccase-catalyzed polymerization of two phenolic compounds studied by matrix-assisted laser desorption/ionization time-of-flight and electrospray ionization Fourier transform ion cyclotron resonance mass spectrometry with collision-induced dissociation experiments. |
| PubMed: | Tropospheric multiphase chemistry of 2,5- and 2,6-dimethylphenols: determination of the mass accommodation coefficients and the Henry's law constants. |
| PubMed: | High-affinity blockade of voltage-operated skeletal muscle sodium channels by 2,6-dimethyl-4-chlorophenol. |
| PubMed: | [Inhibitory effects of phenolic ecotoxicants on photobacteria at various pH values]. |
| PubMed: | Selective oxidative para C-C dimerization of 2,6-dimethylphenol. |
| PubMed: | A comparative study of water soluble 5,10,15,20-tetrakis(2,6-dichloro-3-sulfophenyl)porphyrin and its metal complexes as efficient sensitizers for photodegradation of phenols. |
| PubMed: | Structural features of phenol derivatives determining potency for activation of chloride currents via alpha(1) homomeric and alpha(1)beta heteromeric glycine receptors. |
| PubMed: | Effects of bisphenol A-related diphenylalkanes on vitellogenin production in male carp (Cyprinus carpio) hepatocytes and aromatase (CYP19) activity in human H295R adrenocortical carcinoma cells. |
| PubMed: | Antioxidant activity of propofol and related monomeric and dimeric compounds. |
| PubMed: | [Study on cross-linked capillary columns coated with polyphenylenesiloxane]. |
| PubMed: | Towards a new concept of lignin condensation in kraft pulping. Initial results. |
| PubMed: | Effects of speciation on partitioning of iodine in aqueous biphasic systems and onto ABEC resins. |
| PubMed: | Phenol and lactone receptors in the distal sensilla of the Haller's organ in Ixodes ricinus ticks and their possible role in host perception. |
| PubMed: | Evaluation of the antioxidant properties of propofol and its nitrosoderivative. comparison with homologue substituted phenols. |
| PubMed: | Oxidative polymerization of 2,6-dimethylphenol to form poly(2,6-dimethyl-1,4-phenyleneoxide) in water. |
| PubMed: | Luminescence quenching of tris(2,2'-bipyridine)ruthenium(II) by 2,6-dimethylphenol and 4-bromo-2,6-dimethylphenol in sol-gel-processed silicate thin films. |
| PubMed: | An improved model for the binding of lidocaine and structurally related local anaesthetics to fast-inactivated voltage-operated sodium channels, showing evidence of cooperativity. |
| PubMed: | Depolymerization of poly(2,6-dimethyl-1,4-phenylene oxide) under oxidative conditions. |
| PubMed: | Molybdenum-phosphorus triple bond stabilization by ancillary alkoxide ligation: synthesis and structure of a terminal phosphide tris-1-methylcyclohexanoxide complex. |
| PubMed: | Glucuronidation of propofol and its analogs by human and rat liver microsomes. |
| PubMed: | [Preparation and chromatographic characteristics of linear [60] fullerene polysiloxane stationary phase for capillary gas chromatography]. |
| PubMed: | Vibrational analysis of substituted phenols: part II. Transferability of valence force constants. |
| PubMed: | Block of voltage-operated sodium channels by 2,6-dimethylphenol, a structural analogue of lidocaine's aromatic tail. |
| PubMed: | Chiral proton donor reagents: tin tetrachloride--coordinated optically active binaphthol derivatives. |
| PubMed: | Synthesis of Zirconium Aryloxide Complexes Containing Pendent Vinyl Groups. |
| PubMed: | Structural requirements of phenol derivatives for direct activation of chloride currents via GABA(A) receptors. |
| PubMed: | Determination of phenols in soil by supercritical fluid extraction-capillary electrochromatography. |
| PubMed: | Reactivity of "Eu(OiPr)2" with phenols: formation of linear Eu3, square pyramidal Eu5, cubic Eu8, and capped cubic Eu9 polymetallic europium complexes. |
| PubMed: | Estimation of glycated hemoglobin by 2,6-dimethylphenol: Sulphuric acid conventional method. |
| PubMed: | Anaerobic biodegradability of alkylphenols and fuel oxygenates in the presence of alternative electron acceptors. |
| PubMed: | Nitrosation of phenolic compounds: inhibition and enhancement. |
| PubMed: | Copper(II)-catalyzed reactions of activated aromatics. |
| PubMed: | Effect of acid concentration on chromogen formation from hexoses in sulfuric acid-based reactions. |
| PubMed: | Lignin peroxidase-catalyzed oxidation of sulfonated azo dyes generates novel sulfophenyl hydroperoxides. |
| PubMed: | Oxidative and non-oxidative metabolism of 4-iodoanisole by rat liver microsomes. |
| PubMed: | Cometabolic degradation of o-cresol and 2,6-dimethylphenol by Penicillium frequentans Bi 7/2. |
| PubMed: | Modified Jeener solid-echo pulse sequences for the measurement of the proton dipolar spin-lattice relaxation time (T1D) of tissue solid-like macromolecular components. |
| PubMed: | Determination of menthol in honey by gas chromatography. |
| PubMed: | Potential for carboxylation-dehydroxylation of phenolic compounds by a methanogenic consortium. |
| PubMed: | Determination of ten beta-blockers in urine by micellar electrokinetic capillary chromatography. |
| PubMed: | Myeloperoxidase catalysed cooxidative metabolism of methimazole: oxidation of glutathione and NADH by free radical intermediates. |
| PubMed: | Bacterial metabolism of 2,6-xylenol. |
| PubMed: | Laccase-mediated detoxification of phenolic compounds. |
| PubMed: | The metabolism of N-acetyl-3,5-dimethyl-p-benzoquinone imine in isolated hepatocytes involves N-deacetylation. |
| PubMed: | Acute toxicity of cresols, xylenols, and trimethylphenols to Daphnia magna Straus 1820. |
| PubMed: | 2,6-Dimethylphenol: a new metabolite of mexiletine. |
| PubMed: | Fatal xylenol self-poisoning. |
| PubMed: | Uptake of phenol by Trichosporon cutaneum. |
| PubMed: | Evidence for a one-electron mechanism of 2-aminofluorene oxidation by prostaglandin H synthase and horseradish peroxidase. |
| PubMed: | [Occurrence of phenolic compounds in the dust of swine stalls and henhouses]. |
| PubMed: | Reductive oxygenation of carbon tetrachloride: trichloromethylperoxyl radical as a possible intermediate in the conversion of carbon tetrachloride to electrophilic chlorine. |
| PubMed: | [Establishment of the maximum permissible concentration of 2,6-xylenol in the atmosphere]. |
| PubMed: | [Changes in the erythrocyte osmotic resistance of rats as affected by 2,6-dimethylphenol in vivo and in vitro]. |
| PubMed: | Kinetics and mechanism of hydrolysis of labile quaternary ammonium derivatives of tertiary amines. |
| PubMed: | Cutaneous penetration of some hairdyes in the hairless rat. |
| PubMed: | Oxidation of carbon tetrachloride, bromotrichloromethane, and carbon tetrabromide by rat liver microsomes to electrophilic halogens. |
| PubMed: | Chemical structure of the adducts formed by the oxidation of benzidine in the presence of phenols. |
| PubMed: | Solvent effects of flavin electron transfer reactions. |
| PubMed: | Antimycoplasmal activity of dimethylphenols in a tracheal explant culture system. |
| PubMed: | Inhibition of prostaglandin synthesis in mouse 3T3 fibroblasts and human platelets by substituted phenols. |
| PubMed: | [Experimental data on evaluating the toxicity of 2,6-dimethylphenol]. |
| PubMed: | [Combined effects of 2,6-dimethylphenol and methanol]. |
| PubMed: | Reaction of amide homologs. XVI. Alpha-acetamidoalkylation of beta-naphthol and 2,6-xylenol. |
|
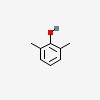 3D/inchi
3D/inchi








