|
Category: flavor and fragrance agents
US / EU / FDA / JECFA / FEMA / FLAVIS / Scholar / Patent Information:
Physical Properties:
| Appearance: | colorless clear liquid (est) |
| Assay: | 99.00 to 100.00 % sum of isomers
|
| Food Chemicals Codex Listed: | Yes |
| Specific Gravity: | 0.84600 to 0.85000 @ 25.00 °C.
|
| Pounds per Gallon - (est).: | 7.040 to 7.073
|
| Specific Gravity: | 0.84600 to 0.85400 @ 20.00 °C.
|
| Pounds per Gallon - est.: | 7.048 to 7.114
|
| Refractive Index: | 1.43600 to 1.45000 @ 20.00 °C.
|
| Boiling Point: | 156.50 °C. @ 760.00 mm Hg
|
| Boiling Point: | 55.00 to 56.00 °C. @ 9.00 mm Hg
|
| Acid Value: | 0.25 max. KOH/g
|
| Vapor Pressure: | 1.039000 mmHg @ 25.00 °C. (est) |
| Vapor Density: | 3.45 ( Air = 1 ) |
| Flash Point: | 142.00 °F. TCC ( 61.11 °C. )
|
| logP (o/w): | 1.697 (est) |
| Shelf Life: | 24.00 month(s) or longer if stored properly. |
| Storage: | store in cool, dry place in tightly sealed containers, protected from heat and light. |
| Soluble in: |
| | alcohol | | | fixed oils | | | propylene glycol | | | water, 1.6e+004 mg/L @ 25 °C (est) |
Organoleptic Properties:
| |
| Odor Type: green |
| |
| Odor Strength: | high ,
recommend smelling in a 10.00 % solution or less |
| |
| Substantivity: | 4 hour(s) at 100.00 % |
| |
| | fresh green grassy foliage vegetable herbal oily |
Odor Description:
at 10.00 % in dipropylene glycol. | fresh green cut grass foliage vegetable herbal oily
Luebke, William tgsc, (1989) |
| |
| | green grassy melon rind pungent |
Odor Description:
| Green, grassy, melon rind-like with a pungent freshness
Mosciano, Gerard P&F 18, No. 4, 51, (1993) |
| |
| |
| Flavor Type: green |
| |
| | fresh green raw fruity pungent |
Taste Description:
at 30.00 ppm. | Fresh, green, raw fruity with a pungent depth
Mosciano, Gerard P&F 18, No. 4, 51, (1993) |
| |
| Odor and/or flavor descriptions from others (if found). |
| |
| Bedoukian Research |
| cis-3-HEXEN-1-OL (LEAF ALCOHOL) ≥98.0%, FCC, Kosher |
| Odor Description: | Powerful, fresh, green grass
Use small amounts for fresh topnotes in delicate floral fragrances such as muguet or lilac. |
| Taste Description: | Characteristic sweet, green, fruity
Adds sweet green notes to all types of fruits, especially apple, pear, peach, and tropical flavors. |
| |
| Firmenich |
| ALEOL™ min. 97%, Kosher, Halal |
| Taste Description: | Powerful fresh, green, grassy and herbaceous notes |
| |
| PerfumersWorld |
| cis-3-Hexenol |
| Odor Description: | fresh natural-green cut-grass leafy Powerful and intensely green grassy
Blends-well-with - Artificial Ess. Oils Galbanum Oakmoss |
| |
| Pell Wall Perfumes |
| cis-3-Hexenol |
| Odor Description: | Green, grassy, foliage. Powerful
According to Arctander: “Traces of cis-3-Hexenol are used in refreshing topnotes in delicate floral fragrance types, such as Muguet and Lilac, and the alcohol is often used along with Geranium oil, Galbanum, Oakmoss, Lavender and Mint oils in various fragrance types.” |
| |
| Robertet |
| Cis-3-hexenol nat 100% Pure & Natural, Kosher |
| Odor Description: | Green, powerful, herbaceous, freshly-cut grass |
| |
| FCI SAS |
| CIS-3-HEXENOL |
| Odor Description: | Powerful and diffusive foliage green odor with typical cutted grass, fresh top note |
| |
| Nagar Haveli Perfumes & Aromatics |
| Cis-3-Hexenol 98% min. Natural |
| Odor Description: | Fresh green cut grass foliage vegetable herbal oily |
| |
| Firmenich |
| CIS-3-HEXENOL NAT Kosher for flavor |
| Odor Description: | Powerful green, fresh, grass and leaf note reminiscent of freshly cut grass |
| Taste Description: | freshly cut grass green and herbaceous notes
Cis-3-HEXENOL NAT is an emblematic compound, so typical of freshly cut herb.
It can be used in all kinds of fruit and vegetable flavors because of its unique green and grassy characteristics |
| |
| |
Cosmetic Information:
Suppliers:
| ACS International |
| Hexenol-cis-3 Nat.
|
| Operational Capabilities |
| ACS International |
| Hexenol-cis-3
|
| Advanced Biotech |
| CIS 3 HEXENOL 92-95% NATURAL
|
| Advanced Biotech |
| CIS 3 HEXENOL NATURAL
97% min. Odor: Green, Leafy |
| Advanced Biotech |
| CIS 3 HEXENOL SYNTHETIC
Odor: Green, Leafy |
| Alfrebro |
| cis-3-HEXENOL NATURAL
Odor: Fresh, Leafy, Green Grass |
| Ambles Nature et Chimie |
| CIS 3 HEXENOL NAT
|
| Apple Flavor & Fragrance |
| cis-3-Hexenol
|
| Aromatic and Allied Chemicals |
| Cis-3-Hexenol
|
| Arora Aromatics |
| CIS-3 - Hexenol ( Natural Cis Hexenol ex Mint )
|
| Artiste |
| cis-3-Hexenol, Natural
|
| Astral Extracts |
| Cis-3-Hexenol
(75%; 65%; 95%) |
| Augustus Oils |
| Cis 3 Hexenol
|
| Services |
| Aurochemicals |
| cis-3-HEXENOL, Natural
|
| Axxence Aromatic |
| CIS-3-HEXENOL 98%, Natural
Kosher |
| Sustainability |
| Bedoukian Research |
| cis-3-HEXEN-1-OL (LEAF ALCOHOL)
≥98.0%, FCC, Kosher Odor: Powerful, fresh, green grass Use: Use small amounts for fresh topnotes in delicate floral fragrances such as muguet or lilac. Flavor: Characteristic sweet, green, fruity Adds sweet green notes to all types of fruits, especially apple, pear, peach, and tropical flavors. |
| Bell Flavors & Fragrances |
| Natural Cis 3 Hexenol 90%
|
| Berjé |
| cis-3-Hexenol Natural
|
| Media |
| Berjé |
| cis-3-Hexenol
|
| BOC Sciences |
| For experimental / research use only. |
| cis-3-HEXEN-1-OL (LEAF ALCOHOL) FCC 98.0%
|
| Charabot |
| Cis-3-hexenol nat
100% Pure & Natural, Kosher |
| Charabot |
| Cis-3-hexenol
Natural identical, Kosher Odor: Green, powerful |
| Charkit Chemical |
| HEXENOL, CIS-3- FEMA 2563
|
| Citrus and Allied Essences |
| cis-3-Hexenol (98%) (natural)
|
| Market Report |
| Citrus and Allied Essences |
| cis-3-Hexenol FCC
|
| CJ Latta & Associates |
| CIS-3-HEXENOL
|
| CJ Latta & Associates |
| Natural Cis-3-hexenol
|
| Creatingperfume.com |
| Hexenol-cis-3-FCC
Odor: grass and fresh green leaves |
| De Monchy Aromatics |
| cis-3-hexenol EU natural
|
| De Monchy Aromatics |
| cis-3-Hexenol
|
| Diffusions Aromatiques |
| Cis-3-HEXENOL NATUREL
|
| Diffusions Aromatiques |
| cis-3-HEXENOL
|
| ECSA Chemicals |
| CIS-3 HEXENOL FLAVOUR USE
|
| ECSA TRADE THE MOST UPDATED FINANCIAL PUBLICATION ON THE WORLD OF CHEMISTRY |
| ECSA Chemicals |
| CIS-3 HEXENOL
|
| EMD Millipore |
| For experimental / research use only. |
| cis-3-Hexen-1-ol
|
| Ernesto Ventós |
| CIS-3-HEXENOL NATURAL
|
| Ernesto Ventós |
| CIS-3-HEXENOL NEGEV FIRMENICH
|
| Ernesto Ventós |
| CIS-3-HEXENOL SUBSTITUTE
|
| Ernesto Ventós |
| CIS-3-HEXENOL ZEON
|
| Ernesto Ventós |
| CIS-3-HEXENOL
|
| Excellentia International |
| cis-3-Hexenol (Leaf Alcohol)
|
| Excellentia International |
| cis-3-Hexenol Natural
|
| FCI SAS |
| CIS-3-HEXENOL NATURAL EU
Odor: Powerful and diffusive foliage green odor with typical cutted grass, fresh top note |
| FCI SAS |
| CIS-3-HEXENOL
Odor: Powerful and diffusive foliage green odor with typical cutted grass, fresh top note |
| Firmenich |
| ALEOL™
min. 97%, Kosher, Halal Flavor: Powerful fresh, green, grassy and herbaceous notes |
| Firmenich |
| CIS-3-HEXENOL NAT Kosher
for flavor Flavor: freshly cut grass green and herbaceous notes Cis-3-HEXENOL NAT is an emblematic compound, so typical of freshly cut herb.
It can be used in all kinds of fruit and vegetable flavors because of its unique green and grassy characteristics |
| Firmenich |
| CIS-3-HEXENOL NAT
for fragrance Odor: Powerful green, fresh, grass and leaf note reminiscent of freshly cut grass Use: Natural molecule obtained by biotechnology.
Indispensable to bring natural green freshness in all kinds of fragrances as it is widely found in nature. |
| Fleurchem |
| cis-3-hexenol natural
|
| Foreverest Resources |
| cis-3-Hexenol 98%
Odor: green grassy Use: cis-3-Hexen-1-ol is also called (Z)-3-hexen-1-ol and leaf alcohol. It is a colorless oily liquid with an intense grassy-green odor of freshly cut green grass and leaves. cis-3-Hexen-1-ol is produced in small amounts by most plants. cis-3-Hexen-1-ol is commonly used as raw material of flavor and frgrance. |
| Gem Aromatics |
| CIS 3 Hexanol Natural 95% & 98%
|
| Global Essence |
| CIS 3 Hexanol
|
| Grau Aromatics |
| HEXEN-3-OL-1, cis
|
| IFF |
| cis-3-Hexenol
Odor: Strong, fresh green grass |
| Ind-Swift Laboratories |
| Cis-3-Hexenol
|
| Indenta Group |
| cis-3-Hexenol
|
| Indukern F&F |
| CIS-3-HEXENOL NATURAL
Odor: STRONG, FRESH, GREEN, GRASS |
| Indukern F&F |
| CIS-3-HEXENOL
|
| Inoue Perfumery |
| CIS-3-HEXENOL
|
| Kingchem Laboratories |
| BETA GAMMA HEXENOL 50/50
Odor: An almost perfect green leaf |
| Kingchem Laboratories |
| C3 HEXENOL (LEAF ALCOHOL)
Odor: Foliage green odor, fresh cut grass top note |
| Lluch Essence |
| CIS-3-HEXENOL 98%
|
| Lluch Essence |
| CIS-3-HEXENOL NATURAL
|
| M&U International |
| Cis 3-Hexenol, Kosher
|
| M&U International |
| NATcis-3-HEXEN-1-OL
|
| Mane |
| Cis-3 Hexenol
Isolate ex Cornmint Odor: Green Grassy Fruity |
| Mentha & Allied Products |
| cis-3-Hexenol
90.00 - 95.00 % Minimum ( Cis-3-Hexenol Content) |
| Moellhausen |
| cis-3-HEXENOL 98%
Odor: strong, fresh, natural, green Flavor: fat, stinging, fresh |
| Moellhausen |
| CIS-3-HEXENOL NAT.
|
| Nagar Haveli Perfumes & Aromatics |
| Cis-3-Hexenol 98% min.
Natural Odor: Fresh green cut grass foliage vegetable herbal oily |
| Naturamole |
| cis-3-hexenol 98% natural EU
|
| Nectar Lifesciences |
| Cis 3 Hexenol
|
| PCW France |
| Cis-3-Hexenol
|
| Steps to a fragranced product |
| Pearlchem Corporation |
| CIS-3-Hexenol
|
| Pell Wall Perfumes |
| cis-3-Hexenol
Odor: Green, grassy, foliage. Powerful Use: According to Arctander: “Traces of cis-3-Hexenol are used in refreshing topnotes in delicate floral fragrance types, such as Muguet and Lilac, and the alcohol is often used along with Geranium oil, Galbanum, Oakmoss, Lavender and Mint oils in various fragrance types.” |
| Penta International |
| CIS-3-HEXENOL FCC
|
| Penta International |
| CIS-3-HEXENOL NATURAL
|
| Penta International |
| CIS-3-HEXENOL
|
| Perfumer Supply House |
| Cis-3-Hexenol aka Leaf Alcohol
Odor: A versatile green, characteristic grass note Use: excellent addition to strawberry and raspberry and other fruity fragrances. |
| PerfumersWorld |
| cis-3-Hexenol 10% in DPG
|
| PerfumersWorld |
| cis-3-Hexenol
Odor: fresh natural-green cut-grass leafy Powerful and intensely green grassy Use: Blends-well-with - Artificial Ess. Oils Galbanum Oakmoss |
| Phoenix Aromas & Essential Oils |
| Cis-3-Hexenol Natural
|
| Phoenix Aromas & Essential Oils |
| Cis-3-Hexenol
|
| Primechem |
| Cis 3 Hexenol
|
| Prinova |
| Cis 3 Hexenol Natural
|
| Prinova |
| CIS 3 Hexenol SYNTHETIC
|
| Prodasynth |
| CIS-3-HEXENOL, NATURAL
(> 97%) Odor: INTENSE, GREEN |
| Prodasynth |
| CIS-3-HEXENOL
(> 98%) Odor: INTENSE, GREEN |
| Quimdis |
| Cis-3-Hexenol Natural
|
| R C Treatt & Co Ltd |
| cis-3-Hexenol
|
| Reincke & Fichtner |
| cis-3-Hexen-1-ol natural
|
| Reincke & Fichtner |
| cis-3-Hexen-1-ol
|
| Riverside Aromatics |
| cis -3-HEXENOL NATURAL
|
| Robertet |
| Cis-3-hexenol nat
100% Pure & Natural, Kosher Odor: Green, powerful, herbaceous, freshly-cut grass |
| Seasons and Harvest / Crop calendar |
| Robertet |
| Cis-3-hexenol
Natural identical, Kosher Odor: Green, powerful |
| Robertet |
| HEXENOL CIS-3
Pure & Nat (EU) |
| Sigma-Aldrich |
| cis-3-Hexen-1-ol, ≥98%, FCC, FG
Odor: green |
| Certified Food Grade Products |
| Sigma-Aldrich |
| cis-3-Hexen-1-ol, natural, >98%, FCC, FG
Odor: green |
| Silverline Chemicals |
| Cis-3-Hexenol(90-98%)
|
| Som Extracts |
| CIS-3-HEXENOL 50 TO 98% NATURAL
|
| SRS Aromatics |
| CIS-3-HEXENOL
|
| Sunaux International |
| cis-3-Hexenol
|
| Sunaux International |
| nat.cis-3-Hexenol
|
| Synerzine |
| cis-2-Hexen-1-ol
|
| Synerzine |
| CIS-3-HEXEN-1-OL (ADDITIVE FREE)
|
| Synerzine |
| cis-3-Hexen-1-ol
|
| Synerzine |
| Natural cis-3-Hexen-1-ol
|
| Taytonn ASCC |
| Cis-3-Hexen-1-ol
Odor: Fresh, Grass, Green, Herbal/ Herbaceous |
| Taytonn ASCC |
| Cis-3-Hexenol
Odor: Fresh, Grass, Green |
| TCI AMERICA |
| For experimental / research use only. |
| cis-3-Hexen-1-ol >97.0%(GC)
|
| Tengzhou Jitian Aroma Chemiclal |
| Cis-3-Hexenol
|
| The John D. Walsh Company |
| Cis-3-Hexenol
|
| The Lermond Company |
| CIS 3 HEXENOL, NATURAL
|
| The Lermond Company |
| CIS 3 HEXENOL
|
| The Perfumers Apprentice |
| Hexenol-3-Cis
Odor: foliage green fresh oily cut grass |
| United International |
| cis-3-Hexenol Nat.
|
| Vigon International |
| Hexenol cis-3 FCC (Leaf Alcohol)
Odor: POWERFUL, GRASSY-GREEN |
| Vigon International |
| Hexenol cis-3 Natural 97% (Leaf Alcohol)
Odor: POWERFUL, GRASSY-GREEN |
| WEN International |
| CIS-3-HEXENOL Natural
|
| Zanos |
| Cis-3-hexenol
Odor: Powerful and intensely green, grassy |
| ZEON Chemicals |
| cis-3-Hexenol
|
Safety Information:
| Preferred SDS: View |
| European information : |
| Most important hazard(s): | | Xi - Irritant |
R 10 - Flammable.
R 36 - Irritating to eyes.
S 02 - Keep out of the reach of children.
S 16 - Keep away from sources of ignition - No Smoking.
S 36 - Wear suitable protective clothing.
|
| |
| Hazards identification |
| |
| Classification of the substance or mixture |
| GHS Classification in accordance with 29 CFR 1910 (OSHA HCS) |
Flammable liquids (Category 3), H226
|
| GHS Label elements, including precautionary statements |
| |
| Pictogram |  |
| |
| Signal word | Warning |
| Hazard statement(s) |
H226 - Flammable liquid and vapour
|
| Precautionary statement(s) |
P210 - Keep away from heat/sparks/open flames/hot surfaces. — No smoking.
P223 - Keep away from any possible contact with water, because of violent reaction and possible flash fire.
P240 - Ground/bond container and receiving equipment.
P241 - Use explosion-proof electrical/ventilating/lighting/…/equipment.
P242 - Use only non-sparking tools.
P243 - Take precautionary measures against static discharge.
P280 - Wear protective gloves/protective clothing/eye protection/face protection.
P303 + P361 + P353 - IF ON SKIN (or hair): Remove/Take off Immediately all contaminated clothing. Rinse SKIN with water/shower.
P370 + P378 - In case of fire: Use dry sand, dry chemical or alcohol-resistant foam for extinction.
P403 + P235 - Store in a well-ventilated place. Keep cool.
P501 - Dispose of contents/ container to an approved waste disposal plant.
|
| Human Experience: |
| 4 % solution: no irritation or sensitization. |
| Oral/Parenteral Toxicity: |
gavage-mouse LD50 [sex: M] 7000 mg/kg
(Gaunt et al., 1969)
gavage-mouse LD50 [sex: F] 7200 mg/kg
(Gaunt et al., 1969)
oral-rat LD50 [sex: M/F] 4700 mg/kg
(Moreno, 1973b)
gavage-rat LD50 [sex: M] 10100 mg/kg
(Gaunt et al., 1969)
gavage-rat LD50 [sex: F] 7300 mg/kg
(Gaunt et al., 1969)
intraperitoneal-mouse LD50 400 mg/kg
Food and Cosmetics Toxicology. Vol. 7, Pg. 451, 1969.
oral-mouse LD50 7000 mg/kg
Food and Cosmetics Toxicology. Vol. 7, Pg. 451, 1969.
intraperitoneal-rat LD50 600 mg/kg
Food and Cosmetics Toxicology. Vol. 7, Pg. 451, 1969.
oral-rat LD50 4700 mg/kg
Food and Cosmetics Toxicology. Vol. 12, Pg. 909, 1974.
|
| Dermal Toxicity: |
skin-rabbit LD50 > 5000 mg/kg
Food and Cosmetics Toxicology. Vol. 12, Pg. 909, 1974.
|
| Inhalation Toxicity: |
|
Not determined
|
Safety in Use Information:
| Category: | flavor and fragrance agents |
| RIFM Fragrance Material Safety Assessment: Search |
| IFRA Code of Practice Notification of the 49th Amendment to the IFRA Code of Practice |
| Recommendation for (Z)-3-hexen-1-ol usage levels up to: | | | 8.0000 % in the fragrance concentrate.
|
| |
| Maximised Survey-derived Daily Intakes (MSDI-EU): | 3700.00 (μg/capita/day) |
| Structure Class: | I |
| Use levels for FEMA GRAS flavoring substances on which the FEMA Expert Panel based its judgments that the substances are generally recognized as safe (GRAS). |
| The Expert Panel also publishes separate extensive reviews of scientific information on all FEMA GRAS flavoring substances and can be found at FEMA Flavor Ingredient Library |
| publication number: 3 |
| Click here to view publication 3 |
| | average usual ppm | average maximum ppm |
| baked goods: | - | 5.00000 |
| beverages(nonalcoholic): | - | 1.00000 |
| beverages(alcoholic): | - | - |
| breakfast cereal: | - | - |
| cheese: | - | - |
| chewing gum: | - | - |
| condiments / relishes: | - | - |
| confectionery froastings: | - | - |
| egg products: | - | - |
| fats / oils: | - | - |
| fish products: | - | - |
| frozen dairy: | - | 3.70000 |
| fruit ices: | - | 3.70000 |
| gelatins / puddings: | - | - |
| granulated sugar: | - | - |
| gravies: | - | - |
| hard candy: | - | 5.00000 |
| imitation dairy: | - | - |
| instant coffee / tea: | - | - |
| jams / jellies: | - | - |
| meat products: | - | - |
| milk products: | - | - |
| nut products: | - | - |
| other grains: | - | - |
| poultry: | - | - |
| processed fruits: | - | - |
| processed vegetables: | - | - |
| reconstituted vegetables: | - | - |
| seasonings / flavors: | - | - |
| snack foods: | - | - |
| soft candy: | - | - |
| soups: | - | - |
| sugar substitutes: | - | - |
| sweet sauces: | - | - |
Safety References:
| European Food Safety Athority(EFSA): | Flavor usage levels; Subacute, Subchronic, Chronic and Carcinogenicity Studies; Developmental / Reproductive Toxicity Studies; Genotoxicity Studies... |
| European Food Safety Authority (EFSA) reference(s): |
Opinion of the Scientific Panel on Food Additives, Flavourings, Processing Aids and Materials in contact with Food (AFC) on a request from the Commission related to Flavouring Group Evaluation 5: Esters of 23 branched- and straight-chain aliphatic saturated primary alcohols and of one secondary alcohol, and 24 branched- and straight-chain unsaturated carboxylic acids from chemical groups 1, 2, and 5
View page or View pdf |
Flavouring Group Evaluation 6, Revision 1 (FGE.06Rev1) - Opinion of the Scientific Panel on Food Additives, Flavourings, Processing Aids and Materials in contact with Food (AFC)
View page or View pdf |
Flavouring Group Evaluation 1, Revision 1 (FGE.01Rev 1): Branched-chain aliphatic saturated aldehydes, carboxylic acids and related esters of primary alcohols and branched-chain carboxylic acids from chemical groups 1 and 2 (Commission Regulation (EC) No 1565/2000 of 18 July 2000) - Scientific Opinion of the Panel on Food Additives, Flavourings, Processing Aids and Materials in contact with Food (AFC) on a request from the Commission
View page or View pdf |
Flavouring Group Evaluation 5, Revision 1 (FGE.05Rev1):Esters of branched- and straight-chain aliphatic saturated primary alcohols and of one secondary alcohol, and branched- and straight-chain unsaturated carboxylic acids from chemical groups 1, 2, and 5 (Commission Regulation (EC) No 1565/2000 of 18 July 2000) [1] - Opinion of the Scientific Panel on Food Additives, Flavourings, Processing Aids and Materials in contact with Food (AFC)
View page or View pdf |
Flavouring Group Evaluation 2, Revision 1 (FGE.02) : Branched- and straight-chain aliphatic saturated primary alcohols and related esters of primary alcohols and straight-chain carboxylic acids and one straight-chain aldehyde from chemical groups 1 and 2 (Commission Regulation (EC) No 1565/2000 of 18 July 2000) - Opinion of the Scientific Panel on Food Additives, Flavourings, Processing Aids and Materials in contact with Food (AFC)
View page or View pdf |
Flavouring Group Evaluation 20, Revision 1 (FGE.20Rev1): Benzyl alcohols, benzaldehydes, a related acetal, benzoic acids and related esters from chemical group 23
View page or View pdf |
Scientific Opinion on Flavouring Group Evaluation 20, Revision 2 (FGE.20Rev2): Benzyl alcohols, benzaldehydes, a related acetal, benzoic acids, and related esters from chemical groups 23 and 30
View page or View pdf |
Flavouring Group Evaluation 5, Revision 2 (FGE.05Rev2): Branched- and straight-chain unsaturated carboxylic acids and esters of these with aliphatic saturated alcohols from chemical groups 1, 2, 3 and 5
View page or View pdf |
Scientific Opinion on Flavouring Group Evaluation 20, Revision 3(FGE.20Rev3): Benzyl alcohols, benzaldehydes, a related acetal, benzoic acids, and related esters from chemical groups 23 and 30
View page or View pdf |
Safety and efficacy of non-conjugated and accumulated unsaturated straight-chain and branched-chain, aliphatic primary alcohols, aldehydes, acids, acetals and esters belonging to chemical group 4 when used as flavourings for all animal species
View page or View pdf |
| EPI System: | View |
| AIDS Citations: | Search |
| Cancer Citations: | Search |
| Toxicology Citations: | Search |
| EPA Substance Registry Services (TSCA): | 928-96-1 |
| EPA ACToR: | Toxicology Data |
| EPA Substance Registry Services (SRS): | Registry |
| Laboratory Chemical Safety Summary : | 5281167 |
| National Institute of Allergy and Infectious Diseases: | Data |
| WISER: | UN 1987 |
| WGK Germany: | 1 |
| | (Z)-hex-3-en-1-ol |
| Chemidplus: | 0000928961 |
| RTECS: | MP8400000 for cas# 928-96-1 |
References:
Other Information:
Potential Blenders and core components note
| |
| For Odor |
| aldehydic |
| aldehydic |
| | citrus carbaldehyde | FR |
| | dodecanal (aldehyde C-12 lauric) | FL/FR |
| | nonanal (aldehyde C-9) | FL/FR |
| | octanal (aldehyde C-8) | FL/FR |
| alliaceous |
| | dibutyl sulfide | FL/FR |
| animal |
| iso | butyl quinoline | FR |
| para- | cresyl caprylate | FL/FR |
| anise |
| | anise seed oil colombia | FL/FR |
| anisic |
| | ocimum basilicum herb oil | FL/FR |
| balsamic |
| iso | amyl benzoate | FL/FR |
| | clover nitrile | FR |
| | fir balsam absolute | FR |
| | fir needle oil siberia | FL/FR |
| bitter |
| | gentian absolute | FL/FR |
| burnt |
| | lepidine | FL/FR |
| citrus |
| | bergamot oil | FL/FR |
| | dihydromyrcenol | FL/FR |
| | grapefruit oil c.p. california | FL/FR |
| | grapefruit pentanol | FR |
| | lemongrass oil | FL/FR |
| | lime oil distilled mexico | FL/FR |
| | litsea cubeba fruit oil | FL/FR |
| | litsea cubeba oil terpeneless | FL/FR |
| | methyl heptenone | FL/FR |
| | tetrahydromyrcenol | FR |
| 10- | undecen-1-ol | FL/FR |
| fatty |
| 5- | methyl-5-hexen-2-one | FL/FR |
| floral |
| iso | amyl salicylate | FL/FR |
| | benzyl acetate | FL/FR |
| | bois de rose oil brazil | FL/FR |
| iso | butyl salicylate | FL/FR |
| | citronellyl acetate | FL/FR |
| | coriander seed oil | FL/FR |
| | cyclamen aldehyde | FL/FR |
| | cyclohexyl ethyl alcohol | FL/FR |
| | dihydroisojasmonate methyl ester | FR |
| | dimethyl benzyl carbinyl butyrate | FL/FR |
| | floral pyranol | FR |
| | gardenia oxide | FR |
| | geranyl acetate | FL/FR |
| | herbal pyran | FR |
| | hexahydrofarnesyl acetone | FL/FR |
| (Z)-3- | hexen-1-yl salicylate | FL/FR |
| alpha- | hexyl cinnamaldehyde | FL/FR |
| | ho leaf oil | FR |
| | hyacinth ether | FR |
| | leerall | FR |
| laevo- | linalool | FL/FR |
| | linalool | FL/FR |
| | linalool oxide | FL/FR |
| | methyl dihydrojasmonate | FL/FR |
| | mimosa absolute | FL/FR |
| | mimosa absolute france | FL/FR |
| | mimosa absolute india | FL/FR |
| | mimosa absolute morocco | FL/FR |
| | muguet carboxaldehyde | FR |
| | narcissus absolute (narcissus tazetta ssp. tazzetta) | FR |
| | nerol | FL/FR |
| | neryl acetate | FL/FR |
| | ocean propanal | FL/FR |
| | orris pyridine 25% IPM | FR |
| | peony alcohol | FR |
| | petitgrain oil paraguay | FL/FR |
| | phenethyl acetate | FL/FR |
| | rose butanoate | FL/FR |
| | tetrahydrolinalool | FL/FR |
| | violet methyl carbonate | FR |
| fruity |
| | allyl amyl glycolate | FR |
| | allyl cyclohexyl propionate | FL/FR |
| iso | amyl butyrate | FL/FR |
| | benzyl propionate | FL/FR |
| | butyl 2-methyl butyrate | FL/FR |
| | diethyl malonate | FL/FR |
| | ethyl heptanoate | FL/FR |
| | green acetate | FR |
| (Z)-3- | hexen-1-yl isobutyrate | FL/FR |
| | hexyl isovalerate | FL/FR |
| | methyl 2-methyl valerate | FL/FR |
| 2- | methyl-2-pentenal | FL/FR |
| | prenyl ethyl ether | FL/FR |
| gamma- | undecalactone (aldehyde C-14 (so-called)) | FL/FR |
| green |
| | acetaldehyde ethyl phenethyl acetal | FL/FR |
| | alfalfa absolute | FR |
| | alfalfa oil | FL/FR |
| | alfalfa resinoid | FL/FR |
| | bark carbaldehyde | FR |
| | birch leaf specialty | FR |
| | butyl heptanoate | FL/FR |
| sec- | butyl-3-methyl but-2-ene thioate | FL/FR |
| | cortex pyridine | FL/FR |
| 3,7- | dimethyl-6-octenoic acid | FL/FR |
| | galbanum oil | FL/FR |
| | green specialty | FR |
| (Z)-3- | hepten-1-ol | FL/FR |
| (Z)-4- | hepten-1-ol | FL/FR |
| 3- | hepten-2-one | FL/FR |
| | heptyl heptanoate | FL/FR |
| | hexanal dihexyl acetal | FL/FR |
| (Z)-3- | hexen-1-yl acetate | FL/FR |
| (Z)-3- | hexen-1-yl benzoate | FL/FR |
| (Z)-3- | hexen-1-yl butyrate | FL/FR |
| (Z)-3- | hexen-1-yl formate | FL/FR |
| (Z)-3- | hexen-1-yl propionate | FL/FR |
| (Z)-3- | hexen-1-yl tiglate | FL/FR |
| (Z)-3- | hexenal | FL/FR |
| | hexyl 2-methyl butyrate | FL/FR |
| | hexyl butyrate | FL/FR |
| | hexyl heptanoate | FL/FR |
| | hexyl hexanoate | FL/FR |
| | hexyl tiglate | FL/FR |
| | hyacinth butanal | FR |
| (Z)- | leaf acetal | FL/FR |
| | lilac acetaldehyde | FL/FR |
| | manzanate (Givaudan) | FL/FR |
| | melon nonenoate | FL/FR |
| | methyl heptine carbonate | FL/FR |
| | methyl octine carbonate | FL/FR |
| 3- | methylthio-4-heptanone | |
| 3,6- | nonadien-1-yl acetate | FL/FR |
| (E,Z)-3,6- | nonadien-1-yl acetate | FL/FR |
| (E,Z)-2,6- | nonadienal | FL/FR |
| (Z)-5- | octen-1-ol | FL/FR |
| | phenyl acetaldehyde | FL/FR |
| | phenyl acetaldehyde dimethyl acetal | FL/FR |
| 3- | phenyl propionaldehyde | FL/FR |
| | propylene acetal | FL/FR |
| (E,E)- | sorbyl acetate | |
| | styralyl acetate | FL/FR |
| 3,5,5- | trimethyl hexanol | FL/FR |
| herbal |
| alpha- | amyl cinnamyl formate | FL/FR |
| | clary sage oil france | FL/FR |
| | clary sage resin america | FR |
| | ilex paraguariensis leaf solid extract | FL/FR |
| | lavender absolute bulgaria | FL/FR |
| | linalyl acetate | FL/FR |
| 5- | methyl-3-heptanone | FL/FR |
| | mistletoe absolute | |
| | oregano specialty | FR |
| alpha- | terpinyl acetate | FL/FR |
| marine |
| green | algae absolute | FL/FR |
| melon |
| | melon carboxaldehyde | FR |
| | watermelon ketone | FR |
| minty |
| (-)-iso | pulegol | FL/FR |
| iso | pulegol | FL/FR |
| | wintergreen oil | FL/FR |
| mossy |
| | oakmoss absolute | FL/FR |
| | oakmoss concrete | FR |
| mushroom |
| 3- | octen-2-ol | FL/FR |
| powdery |
| para- | anisyl acetate | FL/FR |
| | cuminaldehyde | FL/FR |
| 3-(2- | furyl) acrolein | FL/FR |
| black | pepper oil | FL/FR |
| | pimenta acris leaf oil | FL/FR |
| terpenic |
| alpha- | terpineol | FL/FR |
| waxy |
| 1- | dodecanol | FL/FR |
| 2,4- | nonadien-1-ol | FL/FR |
| (Z)-3- | nonen-1-ol | FL/FR |
| woody |
| | cedarwood oil virginia | FR |
| | spruce needle oil canada | FL/FR |
| | woody acetate | FR |
| (Z)- | woody amylene | FR |
| |
| For Flavor |
| |
| No flavor group found for these |
| 2- | acetyl-4-isopropenyl pyridine | FL |
| 2- | acetyl-4-isopropyl pyridine | FL |
| green | algae absolute | FL/FR |
| alpha- | amyl cinnamyl formate | FL/FR |
| | ascorbic acid | FL |
| sec- | butyl-3-methyl but-2-ene thioate | FL/FR |
| 2- | ethyl pyridine | FL |
| 3-(2- | furyl) acrolein | FL/FR |
| | gentian absolute | FL/FR |
| 2- | heptenoic acid | FL |
| | hexanal dihexyl acetal | FL/FR |
| (Z)-3- | hexenoic acid | FL |
| | hexyl heptanoate | FL/FR |
| 3-( | methyl thio) hexanal | FL |
| 2- | methyl-6-propoxypyrazine | FL |
| 3- | methylthio-4-heptanone | |
| | mistletoe absolute | |
| (E,E)-3,5- | octadien-2-one | FL |
| 3- | pentanol | FL |
| | prenyl ethyl ether | FL/FR |
| | propylene acetal | FL/FR |
| 10- | undecen-1-ol | FL/FR |
| aldehydic |
| | nonanal (aldehyde C-9) | FL/FR |
| | octanal (aldehyde C-8) | FL/FR |
| animal |
| para- | cresyl caprylate | FL/FR |
| anise |
| | anise seed oil colombia | FL/FR |
| balsamic |
| | fir needle oil siberia | FL/FR |
| citrus |
| | bergamot oil | FL/FR |
| | grapefruit oil c.p. california | FL/FR |
| | lemongrass oil | FL/FR |
| | lime oil distilled mexico | FL/FR |
| laevo- | linalool | FL/FR |
| | linalool | FL/FR |
| | litsea cubeba fruit oil | FL/FR |
| | nerol | FL/FR |
| alpha- | terpineol | FL/FR |
| cooling |
| iso | butyl salicylate | FL/FR |
| | manzanate (Givaudan) | FL/FR |
| creamy |
| 3- | hepten-2-one | FL/FR |
| gamma- | undecalactone (aldehyde C-14 (so-called)) | FL/FR |
| ethereal |
| 5- | methyl-5-hexen-2-one | FL/FR |
| fatty |
| (Z)-3- | hexen-1-yl benzoate | FL/FR |
| 2,4- | nonadien-1-ol | FL/FR |
| floral |
| | bois de rose oil brazil | FL/FR |
| | citronellyl acetate | FL/FR |
| | dimethyl benzyl carbinyl butyrate | FL/FR |
| 3,7- | dimethyl-6-octenoic acid | FL/FR |
| | lepidine | FL/FR |
| | linalyl acetate | FL/FR |
| | methyl dihydrojasmonate | FL/FR |
| | mimosa absolute morocco | FL/FR |
| | neryl acetate | FL/FR |
| | ocean propanal | FL/FR |
| | tetrahydrolinalool | FL/FR |
| fruity |
| | allyl cyclohexyl propionate | FL/FR |
| iso | amyl benzoate | FL/FR |
| para- | anisyl acetate | FL/FR |
| | benzyl acetate | FL/FR |
| | benzyl propionate | FL/FR |
| | butyl 2-methyl butyrate | FL/FR |
| | butyl heptanoate | FL/FR |
| | diethyl malonate | FL/FR |
| | ethyl heptanoate | FL/FR |
| (Z)-3- | hexen-1-yl isobutyrate | FL/FR |
| | hexyl hexanoate | FL/FR |
| | lilac acetaldehyde | FL/FR |
| | methyl 2-methyl valerate | FL/FR |
| 2- | methyl-2-pentenal | FL/FR |
| 5- | methyl-3-heptanone | FL/FR |
| | rose butanoate | FL/FR |
| | styralyl acetate | FL/FR |
| green |
| | acetaldehyde ethyl phenethyl acetal | FL/FR |
| | alfalfa oil | FL/FR |
| | alfalfa resinoid | FL/FR |
| iso | amyl salicylate | FL/FR |
| | cortex pyridine | FL/FR |
| | cyclamen aldehyde | FL/FR |
| | cyclohexyl ethyl alcohol | FL/FR |
| | dibutyl sulfide | FL/FR |
| | dihydromyrcenol | FL/FR |
| | galbanum oil | FL/FR |
| | geranyl acetate | FL/FR |
| (Z)-3- | hepten-1-ol | FL/FR |
| (Z)-4- | hepten-1-ol | FL/FR |
| | heptyl heptanoate | FL/FR |
| | hexahydrofarnesyl acetone | FL/FR |
| (Z)-3- | hexen-1-yl acetate | FL/FR |
| (Z)-3- | hexen-1-yl butyrate | FL/FR |
| (Z)-3- | hexen-1-yl formate | FL/FR |
| (Z)-3- | hexen-1-yl propionate | FL/FR |
| (Z)-3- | hexen-1-yl salicylate | FL/FR |
| (Z)-3- | hexen-1-yl tiglate | FL/FR |
| (Z)-3- | hexenal | FL/FR |
| | hexyl 2-methyl butyrate | FL/FR |
| | hexyl butyrate | FL/FR |
| | hexyl isovalerate | FL/FR |
| | hexyl tiglate | FL/FR |
| (Z)- | leaf acetal | FL/FR |
| | linalool oxide | FL/FR |
| | melon nonenoate | FL/FR |
| | methyl heptenone | FL/FR |
| | methyl heptine carbonate | FL/FR |
| | methyl octine carbonate | FL/FR |
| (E,Z)-3,6- | nonadien-1-yl acetate | FL/FR |
| 3,6- | nonadien-1-yl acetate | FL/FR |
| (E,Z)-2,6- | nonadienal | FL/FR |
| | oakmoss absolute | FL/FR |
| (Z)-5- | octen-1-ol | FL/FR |
| | phenyl acetaldehyde dimethyl acetal | FL/FR |
| 3- | phenyl propionaldehyde | FL/FR |
| (E,E)- | sorbyl acetate | |
| | sorbyl acetate | FL |
| 3,5,5- | trimethyl hexanol | FL/FR |
| herbal |
| | clary sage oil france | FL/FR |
| | coriander seed oil | FL/FR |
| | ilex paraguariensis leaf solid extract | FL/FR |
| | lavender absolute bulgaria | FL/FR |
| | ocimum basilicum herb oil | FL/FR |
| | petitgrain oil paraguay | FL/FR |
| honey |
| | phenethyl acetate | FL/FR |
| | phenyl acetaldehyde | FL/FR |
| lemon |
| | litsea cubeba oil terpeneless | FL/FR |
| minty |
| (-)-iso | pulegol | FL/FR |
| iso | pulegol | FL/FR |
| | wintergreen oil | FL/FR |
| mushroom |
| 3- | octen-2-ol | FL/FR |
| soapy |
| | dodecanal (aldehyde C-12 lauric) | FL/FR |
| 1- | dodecanol | FL/FR |
| spicy |
| | cumin oleoresin | FL |
| | cuminaldehyde | FL/FR |
| black | pepper oil | FL/FR |
| | pimenta acris leaf oil | FL/FR |
| waxy |
| iso | amyl butyrate | FL/FR |
| alpha- | hexyl cinnamaldehyde | FL/FR |
| | mimosa absolute | FL/FR |
| | mimosa absolute france | FL/FR |
| | mimosa absolute india | FL/FR |
| (Z)-3- | nonen-1-ol | FL/FR |
| | spruce needle oil canada | FL/FR |
| alpha- | terpinyl acetate | FL/FR |
| |
Potential Uses:
Occurrence (nature, food, other): noteSynonyms:
| | aleol (Firmenich) | | | blatteralkohol | | | green leaf alcohol | | (3Z)- | hex-3-en-1-ol | | (Z)- | hex-3-en-1-ol | | cis- | hex-3-en-1-ol | | (Z)- | hex-3-enol | | | hex-3(cis)-en-1-ol | | cis 3- | hexanol | | (3Z)-3- | hexen-1-ol | | (Z)-3- | hexen-1-ol | | beta,gamma- | hexen-1-ol | | cis-3- | hexen-1-ol | | cis-3- | hexen-1-ol (ex Cornmint) | | cis-3- | hexen-1-ol (leaf alcohol) | | cis-3- | hexen-1-ol FCC | | cis-3- | hexen-1-ol natural | | 3- | hexen-1-ol, (3Z)- | | 3- | hexen-1-ol, (Z)- | | 3- | hexen-1-ol, cis- | | (Z)-3- | hexen-1-ol, nature-identical | | | hexen-3-ol-1, cis | | cis-3- | hexene-1-ol | | (Z)-3- | hexenol | | 3-(Z)- | hexenol | | beta-gamma- | hexenol | | cis 3- | hexenol | | cis-3- | hexenol | | C3 | hexenol (leaf alcohol) | | | hexenol cis-3 | | | hexenol cis-3 FCC | | cis-3- | hexenol FCC | | cis-3- | hexenol mixture natural | | cis-3- | hexenol nat | | cis-3- | hexenol nat. | | cis-3- | hexenol natural | | | hexenol-3-cis | | 3- | hexenol, cis- | | cis-3- | hexenol, natural | | | leaf alcohol |
Articles:
| Info: | cis-3-HEXENAL, trans-2-HEXENAL
and 'GREEN GRASS' SMELL |
| PubMed: | Intake and transformation to a glycoside of (Z)-3-hexenol from infested neighbors reveals a mode of plant odor reception and defense. |
| Info: | Volatile Flavor Components in Bogyojosaeng and Suhong Cultivars of Strawberry (Fragaria ananassa Duch.) |
| PubMed: | Olfactory learning and memory in the disease vector mosquito, Aedes aegypti. |
| PubMed: | Three Amino Acid Residues Bind Corn Odorants to McinOBP1 in the Polyembryonic Endoparasitoid of Macrocentrus cingulum Brischke. |
| PubMed: | Chemical studies on curuba (Passiflora mollissima (Kunth) L. H. Bailey) fruit flavour. |
| PubMed: | Soft ionization chemical analysis of secondary organic aerosol from green leaf volatiles emitted by turf grass. |
| PubMed: | Comparison of trap types and colors for capturing emerald ash borer adults at different population densities. |
| PubMed: | Intermittent exposure to traces of green leaf volatiles triggers the production of (Z)-3-hexen-1-yl acetate and (Z)-3-hexen-1-ol in exposed plants. |
| PubMed: | Purification and gas chromatography-combustion-isotope ratio mass spectrometry of aroma compounds from green tea products and comparison to bulk analysis. |
| PubMed: | Atmospheric pressure chemical ionisation mass spectrometry analysis linked with chemometrics for food classification - a case study: geographical provenance and cultivar classification of monovarietal clarified apple juices. |
| PubMed: | Characterization of olfactory receptor neurons for pheromone candidate and plant volatile compounds in the clover root weevil, Sitona lepidus. |
| PubMed: | Early transcriptome analyses of Z-3-Hexenol-treated zea mays revealed distinct transcriptional networks and anti-herbivore defense potential of green leaf volatiles. |
| PubMed: | Flavor of cold-hardy grapes: impact of berry maturity and environmental conditions. |
| PubMed: | Identification of volatile compounds emitted by Artemisia ordosica (Artemisia, Asteraceae) and changes due to mechanical damage and weevil infestation. |
| PubMed: | Highly sensitive electrochemical detection of methyl salicylate using electroactive gold nanoparticles. |
| PubMed: | Development of semiochemical attractants for monitoring and controlling Chlorophorus caragana. |
| PubMed: | Composition of essential oil from aerial and underground parts of Geum rivale and G. urbanum growing in Poland. |
| PubMed: | Effect of nine plant volatiles in the field on the sex pheromones of Leguminivora glycinivorella. |
| PubMed: | Comparative study of the volatiles' composition of healthy and larvae-infested Artemisia ordosica. |
| PubMed: | Attractant and disruptant semiochemicals for Dendroctonus jeffreyi (Coleoptera: Curculionidae: Scolytinae). |
| PubMed: | Aromatically enhanced pear distillates from blanquilla and conference varieties using a packed column. |
| PubMed: | Effects of exposure to plant-derived odorants on behavior and the concentration of stress-related hormones in steers isolated under a novel environment. |
| PubMed: | Towards the development of an autocontamination trap system to manage populations of emerald ash borer (Coleoptera: Buprestidae) with the native entomopathogenic fungus, Beauveria bassiana. |
| PubMed: | Gold nanoparticles-peptide based gas sensor arrays for the detection of food aromas. |
| PubMed: | Changes in the bound aroma profiles of 'Hayward' and 'Hort16A' kiwifruit (Actinidia spp.) during ripening and GC-olfactometry analysis. |
| PubMed: | Characterisation of bound volatile compounds of a low flavour kiwifruit species: Actinidia eriantha. |
| PubMed: | Enhancement of volatile aglycone recovery facilitated by acid hydrolysis of glucosides from Nicotiana flower species. |
| PubMed: | Testing for phytochemical synergism: arthropod community responses to induced plant volatile blends across crops. |
| PubMed: | Characterisation of commercial aromatised vinegars: phenolic compounds, volatile composition and antioxidant activity. |
| PubMed: | Green production of polymer-supported PdNPs: application to the environmentally benign catalyzed synthesis of cis-3-hexen-1-ol under flow conditions. |
| PubMed: | Enhanced attraction of Plutella xylostella (Lepidoptera: Plutellidae) to pheromone-baited traps with the addition of green leaf volatiles. |
| PubMed: | Functional characterizations of chemosensory proteins of the alfalfa plant bug Adelphocoris lineolatus indicate their involvement in host recognition. |
| PubMed: | Environmental and seasonal influences on red raspberry flavour volatiles and identification of quantitative trait loci (QTL) and candidate genes. |
| PubMed: | Volatiles emission patterns in poplar clones varying in response to ozone. |
| PubMed: | Genetic variation in the odorant receptor OR2J3 is associated with the ability to detect the "grassy" smelling odor, cis-3-hexen-1-ol. |
| PubMed: | Species and sexual differences in behavioural responses of a specialist and generalist parasitoid species to host-related volatiles. |
| PubMed: | Electroanalytical studies on green leaf volatiles for potential sensor development. |
| PubMed: | Differential metabolisms of green leaf volatiles in injured and intact parts of a wounded leaf meet distinct ecophysiological requirements. |
| PubMed: | Phytochemicals to suppress Fusarium head blight in wheat-chickpea rotation. |
| PubMed: | The rice hydroperoxide lyase OsHPL3 functions in defense responses by modulating the oxylipin pathway. |
| PubMed: | Aroma chemical composition of red wines from different price categories and its relationship to quality. |
| PubMed: | Functional analysis of general odorant binding protein 2 from the meadow moth, Loxostege sticticalis L. (Lepidoptera: Pyralidae). |
| PubMed: | Effects on 3-mercaptohexan-1-ol precursor concentrations from prolonged storage of Sauvignon blanc grapes prior to crushing and pressing. |
| PubMed: | Field attraction of the vine weevil Otiorhynchus sulcatus to kairomones. |
| PubMed: | Responses of Dendroctonus brevicomis (Coleoptera: Curculionidae) in behavioral assays: implications to development of a semiochemical-based tool for tree protection. |
| PubMed: | Plant volatiles enhance behavioral responses of grapevine moth males, Lobesia botrana to sex pheromone. |
| PubMed: | Aromatic characterization of pot distilled kiwi spirits. |
| PubMed: | Volatiles of French ferns and "fougère" scent in perfumery. |
| PubMed: | Olfaction in dragonflies: electrophysiological evidence. |
| PubMed: | Quality evaluation of olive oil by statistical analysis of multicomponent stable isotope dilution assay data of aroma active compounds. |
| PubMed: | Herbivore-induced volatiles from tea (Camellia sinensis) plants and their involvement in intraplant communication and changes in endogenous nonvolatile metabolites. |
| PubMed: | Comparison of odor-active compounds in grapes and wines from vitis vinifera and non-foxy American grape species. |
| PubMed: | California Lomatiums, Part X. Comparison of composition of the hydrodistilled oils from two subspecies of Lomatium mohavense. |
| PubMed: | Identification of potent odourants in wine and brewed coffee using gas chromatography-olfactometry and comprehensive two-dimensional gas chromatography. |
| PubMed: | Characterization of odor-active compounds of various cherry wines by gas chromatography-mass spectrometry, gas chromatography-olfactometry and their correlation with sensory attributes. |
| PubMed: | Characterization of the bound volatile extract from baby kiwi (Actinidia arguta). |
| PubMed: | Green odor and depressive-like state in rats: toward an evidence-based alternative medicine? |
| PubMed: | Chemical composition, and antioxidant and antimicrobial activities of essential Oil of Phyllostachys heterocycla cv. Pubescens varieties from China. |
| PubMed: | Volatile composition of pomegranates from 9 Spanish cultivars using headspace solid phase microextraction. |
| PubMed: | Mixture of cis-3-hexenol and trans-2-hexenal attenuates behavioral and stress responses induced by 2,5-dihydro-2,4,5-trimethylthiazoline and electric footshock stress in rats. |
| PubMed: | Fusarium infection in maize: volatile induction of infected and neighboring uninfected plants has the potential to attract a pest cereal leaf beetle, Oulema melanopus. |
| PubMed: | Comparison of male and female emerald ash borer (Coleoptera: Buprestidae) responses to phoebe oil and (Z)-3-hexenol lures in light green prism traps. |
| PubMed: | Roles of (Z)-3-hexenol in plant-insect interactions. |
| PubMed: | Specific regulation of pyrethrin biosynthesis in Chrysanthemum cinerariaefolium by a blend of volatiles emitted from artificially damaged conspecific plants. |
| PubMed: | Volatile composition and sensory quality of Spanish pomegranates (Punica granatum L.). |
| PubMed: | Essential oil of Galinsoga parviflora leaves from Colombia. |
| PubMed: | Essential oil of Turnera ulmifolia leaves from Cuba. |
| PubMed: | Cereal crop volatile organic compound induction after mechanical injury, beetle herbivory (Oulema spp.), or fungal infection (Fusarium spp.). |
| PubMed: | Volatile emissions from Alnus glutionosa induced by herbivory are quantitatively related to the extent of damage. |
| PubMed: | Male Phyllotreta striolata (F.) produce an aggregation pheromone: identification of male-specific compounds and interaction with host plant volatiles. |
| PubMed: | Effectiveness of different solid-phase microextraction fibres for differentiation of selected Madeira island fruits based on their volatile metabolite profile--identification of novel compounds. |
| PubMed: | Different patterns of neuronal activities in the infralimbic and prelimbic cortices and behavioral expression in response to two affective odors, 2,5-dihydro-2,4,5-trimethylthiazoline and a mixture of cis-3-hexenol and trans-2-hexenal, in the freely moving rat. |
| PubMed: | [Analysis of essential oil from Mahonia duclouxiana]. |
| PubMed: | Effects of n-hexanal on dopamine release in the striatum of living rats. |
| PubMed: | Leaf volatile emissions of Betula pendula during autumn coloration and leaf fall. |
| PubMed: | Relation between developmental stage, sensory properties, and volatile content of organically and conventionally grown pac choi (Brassica rapa var. Mei Qing Choi). |
| PubMed: | Impact of harvesting and processing conditions on green leaf volatile development and phenolics in Concord grape juice. |
| PubMed: | Identification and quantification of impact aroma compounds in 4 nonfloral Vitis vinifera varieties grapes. |
| PubMed: | Expression of genes associated with aroma formation derived from the fatty acid pathway during peach fruit ripening. |
| PubMed: | Plant volatiles influence electrophysiological and behavioral responses of Lygus hesperus. |
| PubMed: | The tea weevil, Myllocerinus aurolineatus, is attracted to volatiles induced by conspecifics. |
| PubMed: | "Green odor" inhalation by stressed rat dams reduces behavioral and neuroendocrine signs of prenatal stress in the offspring. |
| PubMed: | Immunotoxicity activity of the major essential oil of Filipendula glaberrima against Aedes aegypti L. |
| PubMed: | Evaluating the use of male-produced pheromone components and plant volatiles in two trap designs to monitor Anoplophora glabripennis. |
| PubMed: | Biotic and abiotic factors affect green ash volatile production and emerald ash borer adult feeding preference. |
| PubMed: | Attraction of Anoplophora glabripennis to male-produced pheromone and plant volatiles. |
| PubMed: | Characteristic aroma-active compounds of Korean perilla (Perilla frutescens Britton) leaf. |
| PubMed: | Between plant and diurnal variation in quantities and ratios of volatile compounds emitted by Vicia faba plants. |
| PubMed: | Responses of the crucifer flea beetle to Brassica volatiles in an olfactometer. |
| PubMed: | Essential oil composition of lady's mantle (Alchemilla xanthochlora Rothm.) growing wild in Alpine pastures. |
| PubMed: | Host plant volatiles serve to increase the response of male European grape berry moths, Eupoecilia ambiguella, to their sex pheromone. |
| PubMed: | "Green odor" inhalation by rats down-regulates stress-induced increases in Fos expression in stress-related forebrain regions. |
| PubMed: | Inhibitory effects of Ephedra major Host on Aspergillus parasiticus growth and aflatoxin production. |
| PubMed: | Polymorphism in jasmonate signaling partially accounts for the variety of volatiles produced by Nicotiana attenuata plants in a native population. |
| PubMed: | A key volatile infochemical that elicits a strong olfactory response of the predatory mite Neoseiulus californicus, an important natural enemy of the two-spotted spider mite Tetranychus urticae. |
| PubMed: | Fatty acid derived compounds--the dominant volatile class of the essential oil poor Sonchus arvensis subsp. uliginosus (Bieb.) Nyman. |
| PubMed: | Hydrogen-bond-assisted epoxidation of homoallylic and allylic alcohols with hydrogen peroxide catalyzed by selenium-containing dinuclear peroxotungstate. |
| PubMed: | Essential oil compositions of three Lantana species from Monteverde, Costa Rica. |
| PubMed: | Photooxidation of leaf-wound oxygenated compounds, 1-penten-3-ol, (Z)-3-hexen-1-ol, and 1-penten-3-one, initiated by OH radicals and sunlight. |
| PubMed: | In situ investigation of leaf water status by portable unilateral nuclear magnetic resonance. |
| PubMed: | Oriented responses of grapevine moth larvae Lobesia botrana to volatiles from host plants and an artificial diet on a locomotion compensator. |
| PubMed: | Field-testing of synthetic herbivore-induced plant volatiles as attractants for beneficial insects. |
| PubMed: | Effects of environmental novelty on fear-related behavior and stress responses of rats to emotionally relevant odors. |
| PubMed: | Application of zNosetrade mark for the analysis of selected grape aroma compounds. |
| PubMed: | Analysis of volatiles emitted by potato plants by means of a Colorado beetle electroantennographic detector. |
| PubMed: | Responses of the Mediterranean pine shoot beetle Tomicus destruens (Wollaston) to pine shoot and bark volatiles. |
| PubMed: | Electrophysiological response and attraction of emerald ash borer to green leaf volatiles (GLVs) emitted by host foliage. |
| PubMed: | Identification of volatile compounds used in host location by the black bean aphid, Aphis fabae. |
| PubMed: | Predatory mite attraction to herbivore-induced plant odors is not a consequence of attraction to individual herbivore-induced plant volatiles. |
| PubMed: | Volatile composition in raspberry cultivars grown in the Pacific Northwest determined by stir bar sorptive extraction-gas chromatography-mass spectrometry. |
| PubMed: | Attraction to herbivore-induced plant volatiles by the host-foraging parasitoid fly Exorista japonica. |
| PubMed: | Representation of a mixture of pheromone and host plant odor by antennal lobe projection neurons of the silkmoth Bombyx mori. |
| PubMed: | Serotonergic mediation of the antidepressant-like effect of the green leaves odor in mice. |
| PubMed: | Aroma barrier properties of sodium caseinate-based films. |
| PubMed: | Effects of direct exposure of green odour components on dopamine release from rat brain striatal slices and PC12 cells. |
| PubMed: | Aphid and plant volatiles induce oviposition in an aphidophagous hoverfly. |
| PubMed: | The influence of different nutrient levels on insect-induced plant volatiles in Bt and conventional oilseed rape plants. |
| PubMed: | Functional morphology of antennal chemoreceptors of the parasitoid Microplitis croceipes (Hymenoptera: Braconidae). |
| PubMed: | An antiaphrodisiac in Heliconius melpomene butterflies. |
| PubMed: | Effect of fat level on the perception of five flavor chemicals in ice cream with or without fat mimetics by using a descriptive test. |
| PubMed: | Differential electroantennogram response of females and males of two parasitoid species to host-related green leaf volatiles and inducible compounds. |
| PubMed: | Plants attract parasitic wasps to defend themselves against insect pests by releasing hexenol. |
| PubMed: | Biochemical evaluation of borage (Borago officinalis) rosette leaves through their essential oil and fatty acid composition. |
| PubMed: | Volatile compounds characterizing Tunisian Chemlali and Chétoui virgin olive oils. |
| PubMed: | "Green odor" inhalation reduces the skin-barrier disruption induced by chronic restraint stress in rats: physiological and histological examinations. |
| PubMed: | Cut-induced VOC emissions from agricultural grasslands. |
| PubMed: | Olfactory receptors on the maxillary palps of small ermine moth larvae: evolutionary history of benzaldehyde sensitivity. |
| PubMed: | Tracer aroma compound transfer from a solid and complex-flavored food matrix packed in treated papers or plastic packaging film. |
| PubMed: | Characterization of a BAHD acyltransferase responsible for producing the green leaf volatile (Z)-3-hexen-1-yl acetate in Arabidopsis thaliana. |
| PubMed: | Antifungal activities of major tea leaf volatile constituents toward Colletorichum camelliae Massea. |
| PubMed: | Potent odorants characterize the aroma quality of leaves and stalks in raw and boiled celery. |
| PubMed: | Comparison of volatile constituents of Persicaria odorata(Lour.) Soj√°k (Polygonum odoratum Lour.) and Persicaria hydropiper L. Spach (Polygonum hydropiper L.). |
| PubMed: | Diel periodicity in the production of green leaf volatiles by wild and cultivated host plants of stemborer moths, Chilo partellus and Busseola fusca. |
| PubMed: | [Chemical components of volatiles form withered black poplar leaves with different physiological age]. |
| PubMed: | Volatiles released from bean plants in response to agromyzid flies. |
| PubMed: | Emission of herbivore-induced volatiles in absence of a herbivore--response of Zea mays to green leaf volatiles and terpenoids. |
| PubMed: | Increased EAG responses of tortricid moths after prolonged exposure to plant volatiles: evidence for octopamine-mediated sensitization. |
| PubMed: | Effects of cyclamen mite (Phytonemus pallidus) and leaf beetle (Galerucella tenella) damage on volatile emission from strawberry (Fragaria x ananassa Duch.) plants and orientation of predatory mites (Neoseiulus cucumeris, N. californicus, and Euseius finlandicus). |
| PubMed: | Determination of volatile compounds in grape distillates by solid-phase extraction and gas chromatography. |
| PubMed: | Effects of (Z)-3-hexenol, a major component of green odor, on anxiety-related behavior of the mouse in an elevated plus-maze test and biogenic amines and their metabolites in the brain. |
| PubMed: | Plant-plant signaling: ethylene synergizes volatile emission in Zea mays induced by exposure to (Z)-3-hexen-1-ol. |
| PubMed: | The involvement of volatile infochemicals from spider mites and from food-plants in prey location of the generalist predatory mite Neoseiulus californicus. |
| PubMed: | Developmental and varietal differences in volatile ester formation and acetyl-CoA: alcohol acetyl transferase activities in apple (Malus domestica Borkh.) fruit. |
| PubMed: | Identification of characteristic aroma-active compounds from water dropwort (Oenanthe javanica DC.). |
| PubMed: | Responses to sex pheromone and plant odours by olfactory receptor neurons housed in sensilla auricillica of the codling moth, Cydia pomonella (Lepidoptera: Tortricidae). |
| PubMed: | How rainfall, relative humidity and temperature influence volatile emissions from apple trees in situ. |
| PubMed: | Further field evaluation of synthetic herbivore-induced plant volatiles as attractants for beneficial insects. |
| PubMed: | Volatile C6-aldehydes and Allo-ocimene activate defense genes and induce resistance against Botrytis cinerea in Arabidopsis thaliana. |
| PubMed: | Changes in aroma volatile compounds and ethylene production during "Hujingmilu" peach (Prunus persica L.) fruit development. |
| PubMed: | Chemical composition of corn leaf essential oils and their role in the oviposition behavior of Sesamia nonagrioides females. |
| PubMed: | The role of volatile semiochemicals in mediating host location and selection by nuisance and disease-transmitting cattle flies. |
| PubMed: | Enhancement of attraction to sex pheromones of Spodoptera exigua by volatile compounds produced by host plants. |
| PubMed: | (Z)-3-Hexenol induces defense genes and downstream metabolites in maize. |
| PubMed: | Sensory evaluation of the synergism among odorants present in concentrations below their odor threshold in a Chinese jasmine green tea infusion. |
| PubMed: | EAG and behavioral responses of Helicoverpa armigera males to volatiles from poplar leaves and their combinations with sex pheromone. |
| PubMed: | Effect of fat nature and aroma compound hydrophobicity on flavor release from complex food emulsions. |
| PubMed: | Determination of key odorant compounds in freshly distilled cognac using GC-O, GC-MS, and sensory evaluation. |
| PubMed: | Aroma extract dilution analysis of cv. Meeker (Rubus idaeus L.) red raspberries from Oregon and Washington. |
| PubMed: | Host plant volatiles synergize response to sex pheromone in codling moth, Cydia pomonella. |
| PubMed: | Gas chromatography-olfactometry and chemical quantitative study of the aroma of six premium quality spanish aged red wines. |
| PubMed: | Effect of fungicide residues on the aromatic composition of white wine inoculated with three Saccharomyces cerevisiae strains. |
| PubMed: | Angiosperm bark volatiles disrupt response of Douglas-fir beetle, Dendroctonus pseudotsugae, to attractant-baited traps. |
| PubMed: | Epoxide hydrolase: a mRNA induced by the fungal pathogen Alternaria alternata on rough lemon (Citrus jambhiri Lush). |
| PubMed: | Airborne signals prime plants against insect herbivore attack. |
| PubMed: | Flux of organic compounds from grass measured by relaxed eddy accumulation technique. |
| PubMed: | Characterization of a hydroperoxide lyase gene and effect of C6-volatiles on expression of genes of the oxylipin metabolism in Citrus. |
| PubMed: | Hydroxyaldehyde products from hydroxyl radical reactions of Z-3-hexen-1-ol and 2-methyl-3-buten-2-ol quantified by SPME and API-MS. |
| PubMed: | Activation of the anterior cingulate gyrus by 'Green Odor': a positron emission tomography study in the monkey. |
| PubMed: | The in vitro substrate regiospecificity of recombinant UGT85B1, the cyanohydrin glucosyltransferase from Sorghum bicolor. |
| PubMed: | Aroma composition of Vitis vinifera Cv. tannat: the typical red wine from Uruguay. |
| PubMed: | Volatiles from whitefly-infested plants elicit a host-locating response in the parasitoid, Encarsia formosa. |
| PubMed: | Response of female Cydia molesta (Lepidoptera: Tortricidae) to plant derived volatiles. |
| PubMed: | Psychophysical evaluation of responses to pleasant and mal-odour stimulation in human subjects; adaptation, dose response and gender differences. |
| PubMed: | Volatile ester formation in roses. Identification of an acetyl-coenzyme A. Geraniol/Citronellol acetyltransferase in developing rose petals. |
| PubMed: | Induced volatiles in elicitor-treated and rice blast fungus-inoculated rice leaves. |
| PubMed: | Volatile components and aroma active compounds in aqueous essence and fresh pink guava fruit puree (Psidium guajava L.) by GC-MS and multidimensional GC/GC-O. |
| PubMed: | Behavioral and electrophysiological responses of natural enemies to synomones from tea shoots and kairomones from tea aphids, Toxoptera aurantii. |
| PubMed: | Phenol--another cockchafer attractant shared by Melolontha hippocastani Fabr. and M. melolontha L. |
| PubMed: | Alcoholism in cockchafers: orientation of male Melolontha melolontha towards green leaf alcohols. |
| PubMed: | Aroma compound analysis of Eruca sativa (Brassicaceae) SPME headspace leaf samples using GC, GC-MS, and olfactometry. |
| PubMed: | Host plant volatiles synergize responses of sex pheromone-specific olfactory receptor neurons in male Helicoverpa zea. |
| PubMed: | Composition of the volatiles from intact and mechanically pierced tea aphid-tea shoot complexes and their attraction to natural enemies of the tea aphid. |
| PubMed: | Learning of herbivore-induced and nonspecific plant volatiles by a parasitoid, Cotesia kariyai. |
| PubMed: | 1,4-benzoquinone attracts males of Rhizotrogus vernus Germ. |
| PubMed: | Varietal differentiation of red wines in the Valencian region (Spain). |
| PubMed: | Characterization of volatiles in strawberry guava (Psidium cattleianum Sabine) fruit. |
| PubMed: | Volatile compounds from Salix spp. varieties differing in susceptibility to three willow beetle species. |
| PubMed: | Volatile compounds of endophyte-free and infected tall fescue (Festuca arundinacea Schreb.). |
| PubMed: | Plant odor analysis of apple: antennal response of codling moth females to apple volatiles during phenological development. |
| PubMed: | Single and blended maize volatiles as attractants for diabroticite corn rootworm beetles. |
| PubMed: | Olfactory responses of Ips duplicatus from inner Mongolia, China to nonhost leaf and bark volatiles. |
| PubMed: | Free and bound volatile composition and characterization of some glucoconjugates as aroma precursors in melón de olor fruit pulp (Sicana odorifera). |
| PubMed: | Behavioral responses of the diamondback moth, Plutella xylostella, to green leaf volatiles of Brassica oleracea subsp. capitata. |
| PubMed: | Defensive function of herbivore-induced plant volatile emissions in nature. |
| PubMed: | Strategies of a bark beetle, Pityogenes bidentatus, in an olfactory landscape. |
| PubMed: | Analysis of glycosidically bound aroma precursors in tea leaves. 1. Qualitative and quantitative analyses of glycosides with aglycons as aroma compounds. |
| PubMed: | Aroma-active compounds of miniature beefsteakplant (Mosla dianthera Maxim). |
| PubMed: | Olfactory and quantitative analysis of aroma compounds in elder flower (Sambucus nigra L.) drink processed from five cultivars. |
| PubMed: | The effect of a green leaf volatile on host plant finding by larvae of a herbivorous insect. |
| PubMed: | A hairy root culture of melon produces aroma compounds. |
| PubMed: | Aroma chemicals isolated and identified from leaves of Aloe arborescens Mill. Var. Natalensis Berger. |
| PubMed: | (Z)-3-hexenyl and trans-linalool 3,7-oxide beta-primeverosides isolated as aroma precursors from leaves of a green tea cultivar. |
| PubMed: | Effect of Types of Perfume Compounds on the Hydrophile-Lipophile Balance Temperature. |
| PubMed: | Overexpression of a cytoplasm-localized allene oxide synthase promotes the wound-induced accumulation of jasmonic acid in transgenic tobacco. |
| PubMed: | Insect-based BioFETs with improved signal characteristics. |
| PubMed: | Large-scale preparation of (Z)-3-hexen-1-yl acetate using candida antarctica-immobilized lipase in hexane |
| PubMed: | [Establishment of microanalysis of prostaglandin metabolites by GC/MS and its clinical application]. |
| PubMed: | Genetic manipulation of alcohol dehydrogenase levels in ripening tomato fruit affects the balance of some flavor aldehydes and alcohols |
| PubMed: | [Identification of volatile compounds of hawthorn by gas chromatography/mass spectrometry (GC/MS)]. |
| PubMed: | Expression and characterization of a lepidopteran general odorant binding protein. |
| PubMed: | Green leaf volatiles as antiaggregants for the mountain pine beetle,Dendroctonus ponderosae Hopkins (Coleoptera: Scolytidae). |
| PubMed: | Photosynthetic photon flux, photoperiod, and temperature effects on emissions of (Z)-3-hexenal, (Z)-3-hexenol, and (Z)-3-hexenyl acetate from lettuce. |
| PubMed: | A system and methodology for measuring volatile organic compounds produced by hydroponic lettuce in a controlled environment. |
| PubMed: | Role of plant volatiles in the search for a host by parasitoidDiglyphus isaea (Hymenoptera: Eulophidae). |
| PubMed: | Identification, synthesis, and bioactivity of a male-produced aggregation pheromone in assassin bug,Pristhesancus Plagipennis (Hemiptera: Reduviidae). |
| PubMed: | Temperature increase abolishes ability of turtle olfactory receptors to discriminate similar odorant. |
| PubMed: | Volatile Products of the Lipoxygenase Pathway Evolved from Phaseolus vulgaris (L.) Leaves Inoculated with Pseudomonas syringae pv phaseolicola. |
| PubMed: | Orientation ofMicroplitis croceipes (Hymenoptera: Braconidae) to green leaf volatiles: Dose-response curves. |
| PubMed: | Separation and concentration of delta 17-6-keto-PGF1 alpha using monoclonal antibody to omega 3-olefin structure of trienoic prostanoids. |
| PubMed: | Odor-structure relationships in n-hexenols and n-hexenals. |
| PubMed: | Isolation and identification of allelochemicals that attract the larval parasitoid,Cotesia marginiventris (Cresson), to the microhabitat of one of its hosts. |
| PubMed: | Specificity-related suppression of responses to binary mixtures in olfactory receptors of the Colorado potato beetle. |
| PubMed: | Integration of olfactory information in the Colorado potato beetle brain. |
| PubMed: | Strawberry foliage headspace vapor components at periods of susceptibility and resistance toTetranychus urticae Koch. |
| PubMed: | Odorization of inert gas for occupational safety: psychophysical considerations. |
| PubMed: | Toxicities of host secondary compounds to eggs of theBrassica specialistDasineura brassicae. |
| PubMed: | Progress in synthesis of sensory important trace components of essential oils and natural flavours. |
| PubMed: | [The quantitative composition of natural and technologically changed aromas of plants. IV. Enzymic and thermal reaction products formed during the processing of tomatoes (author's transl)]. |
| PubMed: | Acute (rat and mouse) and short-term (rat) toxicity studies on cis-3-hexen-1-ol. |
|
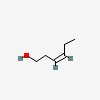 3D/inchi
3D/inchi











































