| Fragrance Demo Formulas Flavor Demo Formulas | |
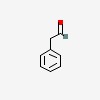
| 2-phenylacetaldehyde (Click)
IUPAC Name:2-phenylacetaldehyde
Std.InChI:InChI=1S/C8H8O/c9-7-6-8-4-2-1-3-5-8/h1-5,7H,6H2
InChI:InChI=1/C8H8O/c9-7-6-8-4-2-1-3-5-8/h1-5,7H,6H2
Std.InChIKey:DTUQWGWMVIHBKE-UHFFFAOYSA-N
InChIKey:DTUQWGWMVIHBKE-UHFFFAOYAO
SMILES:C1=CC=C(C=C1)CC=O
| |
| CAS Number: | 122-78-1 |
| ECHA EC Number: | 204-574-5 |
| FDA UNII: | U8J5PLW9MR |
| Beilstein Number: | 0385791 |
| MDL: | MFCD00006993 |
| FEMA Number: | 2874 |
| CoE Number: | 116 |
| XlogP3: | 1.80 (est) |
| Molecular Weight: | 120.15096000 |
| Formula: | C8 H8 O |
| BioActivity Summary: | listing |
| NMR Predictor: | Predict |
| Category: | flavor and fragrance agents |
| US / EU / FDA / JECFA / FEMA / FLAVIS / Scholar / Patent Information: | |
| Google Scholar: | Search |
| Google Books: | Search |
| Google Scholar: with word "volatile" | Search |
| Google Scholar: with word "flavor" | Search |
| Google Scholar: with word "odor" | Search |
| Perfumer and Flavorist: | Search |
| Google Patents: | Search |
| US Patents: | Search |
| EU Patents: | Search |
| IBM Patents: | Obtain |
| Pubchem Patents: | Search |
| PubMed: | Search |
| NCBI: | Search |
| JECFA Food Flavoring: | 1002 phenylacetaldehyde |
| Flavis Number: | 05.030 (Old) |
| EU SANCO Food Flavourings: | 05.030 phenylacetaldehyde |
| FEMA Number: | 2874 phenylacetaldehyde |
| FDA Mainterm: | PHENYLACETALDEHYDE |
| FDA Regulation: | |
| FDA PART 172 -- FOOD ADDITIVES PERMITTED FOR DIRECT ADDITION TO FOOD FOR HUMAN CONSUMPTION Subpart F--Flavoring Agents and Related Substances Sec. 172.515 Synthetic flavoring substances and adjuvants. | |
| Appearance: | colorless to pale yellow clear oily liquid (est) |
| Assay: | 95.00 to 100.00 % |
| Food Chemicals Codex Listed: | Yes |
| Specific Gravity: | 1.02500 to 1.03500 @ 25.00 °C. |
| Pounds per Gallon - (est).: | 8.529 to 8.612 |
| Refractive Index: | 1.52500 to 1.53200 @ 20.00 °C. |
| Melting Point: | -10.00 °C. @ 760.00 mm Hg |
| Boiling Point: | 193.00 to 195.00 °C. @ 760.00 mm Hg |
| Acid Value: | 5.00 max. KOH/g |
| Odor Type: | green |
| Odor Strength: | high , recommend smelling in a 10.00 % solution or less |
| Odor Description: at 10.00 % in dipropylene glycol. | green sweet floral hyacinth clover honey cocoa Luebke, William tgsc, (1987) |
| Odor sample from: | Berjé Inc. |
| Odor Description: at 2.00 %. | Honey, floral rose, swaat, powdery, fermented, chocolate with a slight earthy nuance Mosciano, Gerard P&F 23, No. 5, 49, (1998) |
| Taste Description: at 5.00 ppm. | Honey, sweet, floral, chocolate and cocoa, with a spicy nuance Mosciano, Gerard P&F 23, No. 5, 49, (1998) |
| Substantivity: | 400 Hour(s) |
| CosIng: | cosmetic data |
| Cosmetic Uses: |
perfuming agents |
| Human Experience: | 2 % solution: skin irritation. sensitising. |
| Oral/Parenteral Toxicity: | |
|
oral-rat LD50 1550 mg/kg (Moreno, 1977m) gavage-guinea pig LD50 [sex: M,F] 3890 mg/kg (Zaitsev & Rakhmanina, 1974) gavage-mouse LD50 [sex: M,F] 3890 mg/kg (Zaitsev & Rakhmanina, 1974) gavage-rat LD50 [sex: M,F] 3890 mg/kg (Zaitsev & Rakhmanina, 1974) oral-guinea pig LD50 3890 mg/kg Voprosy Pitaniya. Problems of Nutrition. Vol. 33(5), Pg. 48, 1974. oral-mouse LD50 3890 mg/kg Voprosy Pitaniya. Problems of Nutrition. Vol. 33(5), Pg. 48, 1974. oral-rat LD50 1550 mg/kg Food and Cosmetics Toxicology. Vol. 17, Pg. 377, 1979. | |
| Dermal Toxicity: | |
|
skin-rabbit LD50 > 5000 mg/kg Food and Cosmetics Toxicology. Vol. 17, Pg. 377, 1979. | |
| Inhalation Toxicity: | |
|
inhalation-mouse LC50 2000 mg/m3 Toksikologicheskii Vestnik. Vol. (2), Pg. 35, 1995. | |
| Category: | flavor and fragrance agents | ||
| Maximised Survey-derived Daily Intakes (MSDI-EU): | 37.00 (μg/capita/day) | ||
| Maximised Survey-derived Daily Intakes (MSDI-USA): | 60.00 (μg/capita/day) | ||
| Structure Class: | I | ||
| Fragrance usage is IFRA RESTRICTED. View Standard for complete information. | |||
| Please review all IFRA documents for complete information. | |||
| IFRA categories: limits in the finished product: (For a description of the categories, refer to the IFRA QRA Information Booklet.) | |||
| Category 1: See Note (1) | 0.02 % (1) | ||
| Category 2: | 0.02 % | ||
| Category 3: | 0.09 % | ||
| Category 4: | 0.30 % | ||
| Category 5: | 0.10 % | ||
| Category 6: | 0.40 % (1) | ||
| Category 7: | 0.04 % | ||
| Category 8: | 0.60 % | ||
| Category 9: | 3.00 % | ||
| Category 10: | 2.50 % | ||
| Category 11: | See Note (2) | ||
| Notes: | |||
(1) IFRA would recommend that any material used to impart perfume or flavour in products intended for human ingestion should consist of ingredients that are in compliance with appropriate regulations for foods and food flavourings in the countries of planned distribution and, where these are lacking, with the recommendations laid down in the Code of Practice of IOFI (International Organisation of the Flavor Industry). Further information about IOFI can be found on its website (www.iofi.org). | |||
(2) Category 11 includes all non-skin contact or incidental skin contact products. Due to the negligible skin contact from these types of products there is no justification for a restriction of the concentration of this fragrance ingredient in the finished product. | |||
| Recommendation for phenyl acetaldehyde flavor usage levels up to: | |||
| 5.0000 ppm in the finished product. | |||
| European Food Safety Athority(efsa): | Flavor usage levels; Subacute, Subchronic, Chronic and Carcinogenicity Studies; Developmental / Reproductive Toxicity Studies; Genotoxicity Studies... |
| European Food Safety Authority (EFSA) reference(s): | |
| Opinion of the Scientific Panel on food additives, flavourings, processing aids and materials in contact with food (AFC) related to: Flavouring Group Evaluation 14 (FGE.14): Phenethyl alcohol, aldehyde, esters, and related phenylacetic acid esters from chemical group 15 (Commission Regulation (EC) No 1565/2000 of 18 July 2000) page or pdf | |
| Flavouring Group Evaluation 55 (FGE.55): Consideration of phenyl-substituted aliphatic alcohols and related aldehydes and esters evaluated by JECFA (63rd meeting) structurally related to phenethyl alcohol, aldehyde, esters and related phenylacetic acid esters evaluated by EFSA in FGE.14 (2005) and aryl-substituted saturated and unsaturated primary alcohol/aldehyde/acid/ester derivatives evaluated by EFSA in FGE.15 (2005) (Commission Regulation (EC) No 1565/2000 of 18 July 2000) - Opinion of the Scientific Panel on Food Additives, Flavourings, Processing Aids and Materials in contact with Food (AFC) page or pdf | |
| Flavouring Group Evaluation 53 (FGE.53): Consideration of phenethyl alcohol, aldehyde, acid and related acetals and esters evaluated by JECFA (59th meeting) structurally related to phenethyl alcohol, aldehyde, esters and related phenylacetic acid esters evaluated by EFSA in FGE.14 (2005) and one phenoxyethyl ester evaluated in FGE.23 (2006) (Commission Regulation (EC) No 1565/2000 of 18 July 2000) - Opinion of the Scientific Panel on Food Additives, Flavourings, Processing Aids and Materials in contact with Food (AFC) page or pdf | |
| Flavouring Group Evaluation 14, Revision 1 (FGE.14Rev1): Phenethyl alcohol, aldehyde, acetals, carboxylic acid and related esters from chemical group 15 and 22 [1] - Opinion of the Scientific Panel on Food Additives, Flavourings, Processing Aids and Materials in Contact with Food page or pdf | |
| Flavouring Group Evaluation 53, Revision 1 (FGE.53Rev1): Consideration of phenethyl alcohol, aldehyde, acid and related acetals and esters evaluated by JECFA (59th meeting) FGE.23Rev1 (2008) page or pdf | |
| EPI System: | View |
| Chemicalize.org: | Calculate predicted properties |
| Cancer Citations: | Search |
| Toxicology Citations: | Search |
| Env. Mutagen Info. Center: | Search |
| EPA Substance Registry Services (TSCA): | 122-78-1 |
| EPA ACToR: | Toxicology Data |
| National Institute of Allergy and Infectious Diseases: | Data |
| SCCNFP: | opinion |
| WGK Germany: | 1 |
| 2-phenylacetaldehyde | |
| DTP/NCI: | 406309 |
| Chemidplus: | 0000122781 |
| RTECS: | CY1420000 for cas# 122-78-1 |
| 2-phenylacetaldehyde | |
| NIST Chemistry WebBook: | Search Inchi |
| Canada Domestic Sub. List: | 122-78-1 |
| Pubchem (cid): | 998 |
| Pubchem (sid): | 134974052 |
| Flavornet: | 122-78-1 |
| Pherobase Floral: | View |
| (IUPAC): | Atomic Weights of the Elements 2009 |
| (IUPAC): | Atomic Weights of the Elements 2009 (pdf) |
| Videos: | The Periodic Table of Videos |
| tgsc: | Atomic Weights use for this web site |
| (IUPAC): | Periodic Table of the Elements |
| FDA Everything Added to Food in the United States (EAFUS): | View |
| CHEBI: | View |
| CHEMBL: | View |
| Golm Metabolome Database: | Search |
| Metabolomics Database: | Search |
| UM BBD: | Search |
| HMDB (Comprehensive Metabolomic Database): | Search |
| Export Tariff Code: | 2912.29.10 |
| VCF-Online: | VCF Volatile Compounds in Food |
| ChemSpider: | View |
| Wikipedia: | View |
| RSC Learn Chemistry: | View |
| acacia cassie farnesiana | FR | |
| aldehydic | FR | |
| artichoke | ||
| blackberry | FR | |
| carnation dianthus oeillet | FR | |
| cherry | FR | |
| cherry blossom | FR | |
| chocolate cocoa | ||
| cinq fleurs forvil | ||
| crabapple blossom | FR | |
| elder berry | FR | |
| evergreen | FR | |
| flower shop | FR | |
| frangipanni plumeria | FR | |
| fresh & clean | FR | |
| gardenia | FR | |
| genet genista broom | FR | |
| grapefruit | FR | |
| green | FR | |
| honey miel | FR | |
| hyacinth jacinthe | FR | |
| jasmin | FR | |
| jockey club | ||
| jonquil narcissus jonquilla | FR | |
| joy | ||
| lilac lilas syringa | FR | |
| lily | FR | |
| magnolia | FR | |
| narcissus narcisse | FR | |
| neroli | FR | |
| orange | FR | |
| peach | FR | |
| pear | FR | |
| powder | FR | |
| pumpkin pie | FR | |
| raspberry | FR | |
| rose | FR | |
| sweet pea | FR | |
| tangerine | FR | |
| tea | ||
| tea green | FR | |
| tobacco | FR | |
| verbena | FR | |
| wallflower | FR |
| allamanda cathartica linn. flower oil brazil @ 0.30% Data GC PbMd GRIN Trop Picture | |
| apple cooked apple | |
| apricot GRIN Trop Picture | |
| asparagus GRIN Trop Picture | |
| bilberry GRIN Trop Picture | |
| blackberry | |
| bread white bread | |
| cabbage | |
| celery leaf GRIN Trop Picture | |
| cerastium candidissimum corr. oil greece @ trace% Data GC GRIN Trop Picture | |
| champaca concrete @ 0.02% Data GC GRIN Trop Picture | |
| cherry | |
| cichorium intybus l. root extract @ 1.04% Data GC GRIN Trop Picture | |
| cider | |
| cocoa GRIN Trop Picture | |
| coriander leaf oil @ 0.03-0.17% Data GC GRIN Trop Picture | |
| eschweilera coriacea (a. p. dc.) mori flower oil brazil @ 2.40% Data GC GRIN Trop Picture | |
| grape | |
| grapefruit juice GRIN Trop Picture | |
| guava GRIN Trop Picture | |
| ham PbMd | |
| herniaria incana lam. oil greece @ 0.20% Data GC GRIN Trop Picture | |
| honey buckwheat honey PbMd | |
| ketaki flower oil india @ 0.40% Data GC GRIN Trop Picture | |
| lecythis usitata miers. var. paraensis (ducke) r. kunth. flower oil brazil @ 28.20% Data GC GRIN Trop Picture | |
| malus sieversii PbMd GRIN Trop Picture | |
| melon | |
| mikania cordata (burm. f.) b.l. robinson var. cordata leaf oil france @ 0.10% Data GC GRIN Trop Picture | |
| narcissus absolute @ 0.15% Data GC GRIN Trop Picture | |
| orange peel GRIN Trop Picture | |
| osmanthus absolute @ trace% Data GC GRIN Trop Picture | |
| papaya | |
| peach | |
| plum | |
| plumcot | |
| potato | |
| pumpkin | |
| raisin | |
| tamarind GRIN Trop Picture | |
| tomato | |
| ylang ylang oil CO2 extract @ 0.03% Data GC GRIN Trop Picture |
| acetaldehyde, phenyl- | |||
| benzacetaldehyde | |||
| benzene acetaldehyde | |||
| benzeneacetaldehyde | |||
| benzyl carboxaldehyde | |||
| benzylcarboxaldehyde | |||
| hyacinthin | |||
| phenyl acetaldehyde natural | |||
| phenyl acetaldehyde pure FCC | |||
| phenyl acetic aldehyde | |||
| phenyl acetic aldehyde 100% | |||
| phenyl ethanal | |||
| 2- | phenyl ethanal | ||
| 1-oxo-2- | phenyl ethane | ||
| oxo | phenyl ethane | ||
| phenyl-acetaldehyde | |||
| phenylacetaldehyd | |||
| phenylacetaldehyde | |||
| 2- | phenylacetaldehyde | ||
| a- | phenylacetaldehyde | ||
| phenylacetaldehyde natural | |||
| phenylacetic aldehyde | |||
| phenylethanal | |||
| 2- | phenylethanal | ||
| 1-oxo-2- | phenylethane | ||
| alpha- | tolualdehyde | ||
| a- | toluic aldehyde | ||
| alpha- | toluic aldehyde | ||
| a- | tolyaldehyde |
click on the picture(s) below to interact with the 3D model | |
| |
| Similar Items: note | |
| para-anisyl acetaldehyde | |
| cumin acetaldehyde | |
| cuminyl acetaldehyde | |
| lilac acetaldehyde | |
| mercaptoacetaldehyde | |
| phenoxyacetaldehyde 50% in benzyl alcohol | |
| phenoxyacetaldehyde 50% in peomosa | |
| pinoacetaldehyde | |
| Soluble in: | |
| alcohol | |
| dipropylene glycol | |
| fixed oils | |
| water, 3026 mg/L @ 25 °C (est) | |
| Insoluble in: | |
| water | |
| glycerin | |
| Stability: | |
| hair spray | |
| non-discoloring in most media | |
| soap | |
















