Articles:
1-phenyl-1-propanol
Notes:
Used as a food additive [EAFUS]
| CAS Number: | 93-54-9 | 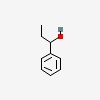 3D/inchi 3D/inchi
|
| ECHA EINECS - REACH Pre-Reg: | 202-256-0 | |
| FDA UNII: | 0F897O3O4M | |
| Nikkaji Web: | J4.678G | |
| Beilstein Number: | 1906759 | |
| MDL: | MFCD00004564 | |
| CoE Number: | 82 | |
| XlogP3: | 1.90 (est) | |
| Molecular Weight: | 136.19384000 | |
| Formula: | C9 H12 O | |
| NMR Predictor: | Predict (works with chrome or firefox) | |
Category: flavor and fragrance agents
US / EU / FDA / JECFA / FEMA / FLAVIS / Scholar / Patent Information:
| Google Scholar: | Search |
| Google Books: | Search |
| Google Scholar: with word "volatile" | Search |
| Google Scholar: with word "flavor" | Search |
| Google Scholar: with word "odor" | Search |
| Perfumer and Flavorist: | Search |
| Google Patents: | Search |
| US Patents: | Search |
| EU Patents: | Search |
| Pubchem Patents: | Search |
| PubMed: | Search |
| NCBI: | Search |
| JECFA Food Flavoring: | 822 1-phenyl-1-propanol |
| DG SANTE Food Flavourings: | 02.033 1-phenylpropan-1-ol |
| FEMA Number: | 2884 1-phenyl-1-propanol |
| FDA: | No longer provide for the use of these seven synthetic flavoring substances |
| FDA Mainterm (SATF): | 93-54-9 ; 1-PHENYL-1-PROPANOL |
| FDA Regulation: | |
| FDA PART 172 -- FOOD ADDITIVES PERMITTED FOR DIRECT ADDITION TO FOOD FOR HUMAN CONSUMPTION Subpart F--Flavoring Agents and Related Substances Sec. 172.515 Synthetic flavoring substances and adjuvants. | |
Physical Properties:
| Appearance: | colorless to pale yellow clear oily liquid (est) |
| Assay: | 97.00 to 100.00 % |
| Additional Assay Information: | racemate |
| Food Chemicals Codex Listed: | No |
| Specific Gravity: | 0.99300 to 1.00000 @ 25.00 °C. |
| Pounds per Gallon - (est).: | 8.263 to 8.321 |
| Refractive Index: | 1.51700 to 1.52200 @ 20.00 °C. |
| Boiling Point: | 103.00 °C. @ 14.00 mm Hg |
| Boiling Point: | 219.00 to 220.00 °C. @ 760.00 mm Hg |
| Vapor Pressure: | 0.071000 mmHg @ 25.00 °C. (est) |
| Flash Point: | 195.00 °F. TCC ( 90.56 °C. ) |
| logP (o/w): | 1.919 (est) |
| Soluble in: | |
| alcohol | |
| water, 0.6 g/L @ 20 °C (exp) | |
| Insoluble in: | |
| water | |
Organoleptic Properties:
| Odor Type: floral | |
| sweet floral balsamic | |
| Odor Description: at 100.00 %. | sweet floral balsam |
| Flavor Type: balsamic | |
| balsamic honey | |
| Taste Description: | balsam honey |
| Odor and/or flavor descriptions from others (if found). | |
Cosmetic Information:
| None found |
Suppliers:
| Aurochemicals |
| PHENYL PROPYL ALCOHOL, Natural |
| BOC Sciences |
| For experimental / research use only. |
| 1-Phenyl-1-propanol |
| Parchem |
| 1-phenyl propyl alcohol |
| Penta International |
| 1-PHENYL-1-PROPANOL |
| Santa Cruz Biotechnology |
| For experimental / research use only. |
| 1-Phenyl-1-propanol |
| Sigma-Aldrich |
| 1-Phenyl-1-propanol, ≥97%, FG
Odor: balsam; floral; sweet |
| Certified Food Grade Products |
| TCI AMERICA |
| For experimental / research use only. |
| 1-Phenyl-1-propanol >98.0%(GC) |
Safety Information:
| Preferred SDS: View | |
| European information : | |
| Most important hazard(s): | |
| Xi - Irritant | |
|
R 38 - Irritating to skin. S 02 - Keep out of the reach of children. S 24 - Avoid contact with skin. S 36 - Wear suitable protective clothing. | |
| Hazards identification | |
| Classification of the substance or mixture | |
| GHS Classification in accordance with 29 CFR 1910 (OSHA HCS) | |
| None found. | |
| GHS Label elements, including precautionary statements | |
| Pictogram | |
| Hazard statement(s) | |
| None found. | |
| Precautionary statement(s) | |
| None found. | |
| Oral/Parenteral Toxicity: | |
|
gavage-rat LD50 2800 mg/kg (Brown et al., 1955) gavage-rat LD50 2500 mg/kg (Rohrbach & Robineau, 1958) gavage-mouse LD50 500 mg/kg (Rohrbach & Robineau, 1958) oral-mouse LD50 500 mg/kg Archives Internationales de Pharmacodynamie et de Therapie. Vol. 116, Pg. 154, 1958. unreported-mouse LD50 1000 mg/kg German Offenlegungsschrift Patent Document. Vol. #2035334 | |
| Dermal Toxicity: | |
|
skin-rabbit LD50 > 2000 mg/kg German Offenlegungsschrift Patent Document. Vol. #2035334 subcutaneous-mouse LD50 700 mg/kg Archives Internationales de Pharmacodynamie et de Therapie. Vol. 116, Pg. 154, 1958. | |
| Inhalation Toxicity: | |
| Not determined | |
Safety in Use Information:
| Category: | flavor and fragrance agents | ||
| RIFM Fragrance Material Safety Assessment: Search | |||
| IFRA Code of Practice Notification of the 49th Amendment to the IFRA Code of Practice | |||
| Recommendation for 1-phenyl propyl alcohol usage levels up to: | |||
| 0.6000 % in the fragrance concentrate. | |||
| Maximised Survey-derived Daily Intakes (MSDI-EU): | 0.24 (μg/capita/day) | ||
| Maximised Survey-derived Daily Intakes (MSDI-USA): | 0.10 (μg/capita/day) | ||
| Threshold of Concern: | 1800 (μg/person/day) | ||
| Structure Class: | I | ||
| Use levels for FEMA GRAS flavoring substances on which the FEMA Expert Panel based its judgments that the substances are generally recognized as safe (GRAS). | |||
| The Expert Panel also publishes separate extensive reviews of scientific information on all FEMA GRAS flavoring substances and can be found at FEMA Flavor Ingredient Library | |||
| publication number: 3 | |||
| Click here to view publication 3 | |||
| average usual ppm | average maximum ppm | ||
| baked goods: | - | 1.50000 | |
| beverages(nonalcoholic): | - | 0.50000 | |
| beverages(alcoholic): | - | - | |
| breakfast cereal: | - | - | |
| cheese: | - | - | |
| chewing gum: | - | - | |
| condiments / relishes: | - | - | |
| confectionery froastings: | - | - | |
| egg products: | - | - | |
| fats / oils: | - | - | |
| fish products: | - | - | |
| frozen dairy: | - | 0.50000 | |
| fruit ices: | - | 0.50000 | |
| gelatins / puddings: | - | - | |
| granulated sugar: | - | - | |
| gravies: | - | - | |
| hard candy: | - | 1.50000 | |
| imitation dairy: | - | - | |
| instant coffee / tea: | - | - | |
| jams / jellies: | - | - | |
| meat products: | - | - | |
| milk products: | - | - | |
| nut products: | - | - | |
| other grains: | - | - | |
| poultry: | - | - | |
| processed fruits: | - | - | |
| processed vegetables: | - | - | |
| reconstituted vegetables: | - | - | |
| seasonings / flavors: | - | - | |
| snack foods: | - | - | |
| soft candy: | - | - | |
| soups: | - | - | |
| sugar substitutes: | - | - | |
| sweet sauces: | - | - | |
Safety References:
| Flavor & Extract Manufacturers Association (FEMA) reference(s): | |
| The FEMA GRAS assessment of aromatic substituted secondary alcohols, ketones, and related esters used as flavor ingredients. View pdf | |
| European Food Safety Athority(EFSA): | Flavor usage levels; Subacute, Subchronic, Chronic and Carcinogenicity Studies; Developmental / Reproductive Toxicity Studies; Genotoxicity Studies... |
| European Food Safety Authority (EFSA) reference(s): | |
| Opinion of the Scientific Panel on food additives, flavourings, processing aids and materials in contact with food (AFC) related to Flavouring Group Evaluation 16 (FGE.16): Aromatic ketones from chemical group 21 (Commission Regulation (EC) No 1565/2000 of 18 July 2000) View page or View pdf | |
| Flavouring Group Evaluation 16, Revision 1 (FGE.16Rev1)[1]: Aromatic ketones from chemical group 21- Opinion of the Scientific Panel on Food Additives, Flavourings, Processing Aids and Materials in Contact with Food View page or View pdf | |
| Flavouring Group Evaluation 69, (FGE.69)[1] - Consideration of aromatic substituted secondary alcohols, ketones and related esters evaluated by JECFA (57th meeting) structurally related to aromatic ketones from chemical group 21 evaluated by EFSA in FGE.16 (2006) - Opinion of the Scientific Panel on Food Additives, Flavourings, Processing Aids and Materials in Contact with Food View page or View pdf | |
| Flavouring Group Evaluation 16, Revision 2 (FGE.16Rev2): Aromatic ketones from chemical group 21 View page or View pdf | |
| Scientific Opinion on Flavouring Group Evaluation 69, Revision 1 (FGE.69Rev1): consideration of aromatic substituted secondary alcohols, ketones and related esters evaluated by JECFA (57th meeting), structurally related to aromatic ketones from chemical group 21 evaluated by EFSA in FGE.16Rev2 View page or View pdf | |
| EPI System: | View |
| AIDS Citations: | Search |
| Cancer Citations: | Search |
| Toxicology Citations: | Search |
| EPA Substance Registry Services (TSCA): | 93-54-9 |
| EPA ACToR: | Toxicology Data |
| EPA Substance Registry Services (SRS): | Registry |
| Laboratory Chemical Safety Summary : | 7147 |
| National Institute of Allergy and Infectious Diseases: | Data |
| WGK Germany: | 3 |
| 1-phenylpropan-1-ol | |
| Chemidplus: | 0000093549 |
| RTECS: | DO5470000 for cas# 93-54-9 |
References:
| 1-phenylpropan-1-ol | |
| NIST Chemistry WebBook: | Search Inchi |
| Canada Domestic Sub. List: | 93-54-9 |
| Pubchem (cid): | 7147 |
| Pubchem (sid): | 134973001 |
Other Information:
| (IUPAC): | Atomic Weights of the Elements 2011 (pdf) |
| Videos: | The Periodic Table of Videos |
| tgsc: | Atomic Weights use for this web site |
| (IUPAC): | Periodic Table of the Elements |
| FDA Substances Added to Food (formerly EAFUS): | View |
| CHEMBL: | View |
| KEGG (GenomeNet): | D01470 |
| HMDB (The Human Metabolome Database): | HMDB31627 |
| FooDB: | FDB008267 |
| Export Tariff Code: | 2906.29.6000 |
| ChemSpider: | View |
Potential Blenders and core components note
Potential Uses:
| acacia | FR | |
| allspice | FR | |
| apricot | FR | |
| balsam | FR | |
| benzoin absolute replacer | FL/FR | |
| blue grass | FR | |
| candy | FL | |
| carnation | FR | |
| cassia | FR | |
| cinnamon | FR | |
| citrus | FR | |
| cloudberry bakeapple | FL | |
| cranberry | FR | |
| cream ice cream | FL | |
| cyclamen | FR | |
| hazelnut | FR | |
| herbal | FR | |
| honey | FR | |
| huckleberry | FR | |
| hyacinth | FR | |
| jasmin | FR | |
| jonquil | FR | |
| leather | FR | |
| lilac | FR | |
| lily of the valley | FR | |
| linden flower | FR | |
| narcissus | FR | |
| orchid | FR | |
| oriental | FR | |
| passion fruit | FR | |
| peach | FR | |
| peru balsam | FR | |
| pistachio | FL | |
| plum | FR | |
| reseda | FR | |
| rose | FR | |
| spice | FR | |
| strawberry | FR | |
| styrax | FR | |
| tea | FL | |
| walnut | FL | |
| ylang ylang | FR |
Occurrence (nature, food, other): note
| not found in nature |
Synonyms:
| benzenemethanol, a-ethyl- | |
| bilergon | |
| carbicol | |
| choleda | |
| ejibil | |
| epatoxfen | |
| alpha- | ethyl benzene methanol |
| alpha- | ethyl benzyl alcohol |
| ethyl phenyl carbinol | |
| a- | ethylbenzenemethanol |
| (±)-a- | ethylbenzyl alcohol |
| a- | ethylbenzyl alcohol |
| ethylphenylcarbinol | |
| felicur | |
| felitrope | |
| fepar | |
| gallenperlen | |
| alpha- | hydroxypropyl benzene |
| livonal | |
| phenyl cholon | |
| phenyl ethyl carbinol | |
| 1- | phenyl propan-1-ol |
| 1- | phenyl propanol |
| 1- | phenyl-1-hydroxypropane |
| 1- | phenyl-1-propanol |
| 1- | phenylpropan-1-ol |
| 1- | phenylpropanol |
| 1- | phenylpropyl alcohol |
| propan-1-ol, 1-phenyl- | |
| unichol |



