Articles:
muguesia (IFF)
Notes:
None found
| Fragrance Demo Formulas | ||
| CAS Number: | 56836-93-2 | 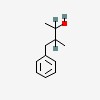 3D/inchi 3D/inchi
|
| ECHA EINECS - REACH Pre-Reg: | 260-398-9 | |
| FDA UNII: | Search | |
| Nikkaji Web: | J267.113A | |
| XlogP3-AA: | 2.70 (est) | |
| Molecular Weight: | 164.24772000 | |
| Formula: | C11 H16 O | |
| NMR Predictor: | Predict (works with chrome, Edge or firefox) | |
Category: fragrance agents
US / EU / FDA / JECFA / FEMA / FLAVIS / Scholar / Patent Information:
| Google Scholar: | Search |
| Google Books: | Search |
| Google Scholar: with word "volatile" | Search |
| Google Scholar: with word "flavor" | Search |
| Google Scholar: with word "odor" | Search |
| Perfumer and Flavorist: | Search |
| Google Patents: | Search |
| US Patents: | Search |
| EU Patents: | Search |
| Pubchem Patents: | Search |
| PubMed: | Search |
| NCBI: | Search |
Physical Properties:
| Appearance: | colorless to pale yellow clear liquid (est) |
| Assay: | 88.00 to 100.00 % |
| Food Chemicals Codex Listed: | No |
| Specific Gravity: | 0.96900 to 0.97700 @ 25.00 °C. |
| Pounds per Gallon - (est).: | 8.063 to 8.130 |
| Refractive Index: | 1.51300 to 1.51900 @ 20.00 °C. |
| Boiling Point: | 256.00 to 257.00 °C. @ 760.00 mm Hg |
| Vapor Pressure: | 0.008000 mmHg @ 25.00 °C. (est) |
| Flash Point: | > 212.00 °F. TCC ( > 100.00 °C. ) |
| logP (o/w): | 2.615 |
| Shelf Life: | 36.00 month(s) or longer if stored properly. |
| Storage: | store in cool, dry place in tightly sealed containers, protected from heat and light. |
| Soluble in: | |
| alcohol | |
| water, 716.5 mg/L @ 25 °C (est) | |
| Insoluble in: | |
| water | |
| Stability: | |
| acid cleaner | |
| alcoholic lotion | |
| antiperspirant | |
| deo stick | |
| detergent | |
| fabric softener | |
| hard surface cleaner | |
| shampoo | |
| soap | |
Organoleptic Properties:
| Odor Type: floral | |
| Odor Strength: | medium |
| Substantivity: | 137 hour(s) at 10.00 % in dipropylene glycol |
| floral muguet green rose mentholic | |
| Odor Description: at 100.00 %. | floral muguet green rose mentholic Luebke, William tgsc, (2009) |
| Odor and/or flavor descriptions from others (if found). | |
| IFF | |
| Muguesia | |
| Odor Description: | Floral muguet comes into its own where aldehydic muguet ingredients are not stable |
| IFF | |
| Starfleur™ 40 | |
| Odor Description: | A fresh, highly performing floral green, muguet, freesia, aldehydic note, with a natural transparent effect Starfleur 40 rounds off green aldehydic muguet notes very well. |
| Azelis UK | |
| STARFLEUR 40 | |
| Odor Description: | A fresh, highly performing floral green, muguet, freesia, aldehydic note, with a natural transparent effect |
Cosmetic Information:
| None found |
Suppliers:
| Associate Allied Chemicals |
| Starfleur 40 |
| About |
| Azelis UK |
| MUGUESIA |
| Azelis UK |
| STARFLEUR 40 |
| Ernesto Ventós |
| MUGUESIA IFF
Odor: FLORAL, MUGUET, ROSY, MINTY |
| Ernesto Ventós |
| STARFLEUR? 40 IFF |
| Fine Fragrances Pvt Ltd |
| Starfleur 40 |
| IFF |
| Muguesia
Odor: Floral muguet comes into its own where aldehydic muguet ingredients are not stable |
| IFF |
| Starfleur™ 40
Odor: A fresh, highly performing floral green, muguet, freesia, aldehydic note, with a natural transparent effect Use: Starfleur 40 rounds off green aldehydic muguet notes very well. |
| M&U International |
| Muguesia(IFF# : 131542) |
| M&U International |
| Starfleur |
| Taytonn ASCC |
| Muguesia |
| The John D. Walsh Company |
| Muguesia |
| Tianjin Danjun International |
| 3-Methyl-4-phenylbutan-2-ol |
Safety Information:
| Hazards identification | |
| Classification of the substance or mixture | |
| GHS Classification in accordance with 29 CFR 1910 (OSHA HCS) | |
| None found. | |
| GHS Label elements, including precautionary statements | |
| Pictogram | |
| Hazard statement(s) | |
| None found. | |
| Precautionary statement(s) | |
| None found. | |
| Oral/Parenteral Toxicity: | |
| Not determined | |
| Dermal Toxicity: | |
| Not determined | |
| Inhalation Toxicity: | |
| Not determined | |
Safety in Use Information:
| Category: | fragrance agents | ||
| RIFM Fragrance Material Safety Assessment: Search | |||
| IFRA Code of Practice Notification of the 49th Amendment to the IFRA Code of Practice | |||
| Recommendation for muguet butanol usage levels up to: | |||
| 15.0000 % in the fragrance concentrate. | |||
| Recommendation for muguet butanol flavor usage levels up to: | |||
| not for flavor use. | |||
Safety References:
| EPI System: | View |
| AIDS Citations: | Search |
| Cancer Citations: | Search |
| Toxicology Citations: | Search |
| EPA Substance Registry Services (TSCA): | 56836-93-2 |
| EPA ACToR: | Toxicology Data |
| EPA Substance Registry Services (SRS): | Registry |
| Laboratory Chemical Safety Summary : | 92543 |
| National Institute of Allergy and Infectious Diseases: | Data |
| 3-methyl-4-phenylbutan-2-ol | |
| Chemidplus: | 0056836932 |
References:
| Leffingwell: | Chirality or Article |
| 3-methyl-4-phenylbutan-2-ol | |
| NIST Chemistry WebBook: | Search Inchi |
| Pubchem (cid): | 92543 |
| Pubchem (sid): | 135048334 |
Other Information:
| (IUPAC): | Atomic Weights of the Elements 2011 (pdf) |
| Videos: | The Periodic Table of Videos |
| tgsc: | Atomic Weights use for this web site |
| (IUPAC): | Periodic Table of the Elements |
| HMDB (The Human Metabolome Database): | Search |
| Export Tariff Code: | 29062920000 |
| ChemSpider: | View |
Potential Blenders and core components note
Potential Uses:
| aldehydic | FR | |
| floral | FR | |
| lily of the valley | FR | |
| rose | FR |
Occurrence (nature, food, other): note
| not found in nature |
Synonyms:
| alpha,beta- | dimethyl benzene propanol |
| 3- | methyl-4-phenyl 2-butanol |
| 3- | methyl-4-phenyl butan-2-ol |
| 3- | methyl-4-phenyl-2-butanol |
| 3- | methyl-4-phenylbutan-2-ol |
| muguesia (IFF) | |
| starfleur 40 (IFF) |






