Articles:
4-phenylbutan-2-ol
Notes:
Used as a food additive [EAFUS]
| CAS Number: | 2344-70-9 | 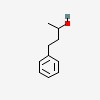 3D/inchi 3D/inchi
|
| ECHA EINECS - REACH Pre-Reg: | 219-055-9 | |
| FDA UNII: | SZ2IE5UDQR | |
| Nikkaji Web: | J1.806F | |
| MDL: | MFCD00044349 | |
| CoE Number: | 85 | |
| XlogP3-AA: | 2.30 (est) | |
| Molecular Weight: | 150.22078000 | |
| Formula: | C10 H14 O | |
| BioActivity Summary: | listing | |
| NMR Predictor: | Predict (works with chrome, Edge or firefox) | |
Category: flavor and fragrance agents
US / EU / FDA / JECFA / FEMA / FLAVIS / Scholar / Patent Information:
| Google Scholar: | Search |
| Google Books: | Search |
| Google Scholar: with word "volatile" | Search |
| Google Scholar: with word "flavor" | Search |
| Google Scholar: with word "odor" | Search |
| Perfumer and Flavorist: | Search |
| Google Patents: | Search |
| US Patents: | Search |
| EU Patents: | Search |
| Pubchem Patents: | Search |
| PubMed: | Search |
| NCBI: | Search |
| JECFA Food Flavoring: | 815 4-phenyl-2-butanol |
| DG SANTE Food Flavourings: | 02.036 4-phenylbutan-2-ol |
| FEMA Number: | 2879 4-phenyl-2-butanol |
| FDA: | No longer provide for the use of these seven synthetic flavoring substances |
| FDA Mainterm (SATF): | 2344-70-9 ; 4-PHENYL-2-BUTANOL |
| FDA Regulation: | |
| FDA PART 172 -- FOOD ADDITIVES PERMITTED FOR DIRECT ADDITION TO FOOD FOR HUMAN CONSUMPTION Subpart F--Flavoring Agents and Related Substances Sec. 172.515 Synthetic flavoring substances and adjuvants. | |
Physical Properties:
| Appearance: | colorless clear oily liquid (est) |
| Assay: | 97.00 to 100.00 % |
| Additional Assay Information: | racemate |
| Food Chemicals Codex Listed: | No |
| Specific Gravity: | 0.97700 to 0.98300 @ 25.00 °C. |
| Pounds per Gallon - (est).: | 8.130 to 8.180 |
| Refractive Index: | 1.51400 to 1.51800 @ 20.00 °C. |
| Boiling Point: | 123.00 to 124.00 °C. @ 15.00 mm Hg |
| Boiling Point: | 239.00 to 240.00 °C. @ 760.00 mm Hg |
| Vapor Pressure: | 0.015000 mmHg @ 25.00 °C. (est) |
| Flash Point: | 231.00 °F. TCC ( 110.56 °C. ) |
| logP (o/w): | 2.131 (est) |
| Soluble in: | |
| alcohol | |
| water, 1885 mg/L @ 25 °C (est) | |
| Insoluble in: | |
| water | |
Organoleptic Properties:
| Odor Type: floral | |
| Odor Strength: | medium |
| Substantivity: | 44 hour(s) at 100.00 % |
| floral peony foliage sweet mimosa heliotrope | |
| Odor Description: at 100.00 %. | floral peony foliage sweet mimosa heliotrope Luebke, William tgsc, (2007) |
| Flavor Type: floral | |
| floral spicy magnolia mango green petal tropical melon | |
| Taste Description: | floral spicy magnolia mango green petal tropical melon Luebke, William tgsc, (2007) |
| Odor and/or flavor descriptions from others (if found). | |
Cosmetic Information:
| None found |
Suppliers:
| Alfrebro |
| 4-PHENYL-2-BUTANOL NATURAL
Odor: Aromatic, Floral |
| BOC Sciences |
| For experimental / research use only. |
| 4-Phenyl-2-butanol |
| EMD Millipore |
| For experimental / research use only. |
| 4-Phenyl-2-butanol |
| Ernesto Ventós |
| 4-PHENYL-2-BUTANOL
Odor: FRUITY, FLORAL |
| Penta International |
| 4-PHENYL-2-BUTANOL |
| Reincke & Fichtner |
| 4-Phenyl-2-butanol |
| Santa Cruz Biotechnology |
| For experimental / research use only. |
| 4-Phenyl-2-butanol ≥98% |
| Sigma-Aldrich: Aldrich |
| For experimental / research use only. |
| 4-Phenyl-2-butanol 97% |
| Synerzine |
| 4-Phenyl-2-butanol |
| TCI AMERICA |
| For experimental / research use only. |
| 4-Phenyl-2-butanol >99.0%(GC) |
Safety Information:
| Preferred SDS: View | |
| European information : | |
| Most important hazard(s): | |
| None - None found. | |
|
S 02 - Keep out of the reach of children. S 24/25 - Avoid contact with skin and eyes. | |
| Hazards identification | |
| Classification of the substance or mixture | |
| GHS Classification in accordance with 29 CFR 1910 (OSHA HCS) | |
| None found. | |
| GHS Label elements, including precautionary statements | |
| Pictogram | |
| Hazard statement(s) | |
| None found. | |
| Precautionary statement(s) | |
| None found. | |
| Oral/Parenteral Toxicity: | |
| Not determined | |
| Dermal Toxicity: | |
| Not determined | |
| Inhalation Toxicity: | |
| Not determined | |
Safety in Use Information:
| Category: | flavor and fragrance agents | ||
| RIFM Fragrance Material Safety Assessment: Search | |||
| IFRA Code of Practice Notification of the 49th Amendment to the IFRA Code of Practice | |||
| Recommendation for 4-phenyl-2-butanol usage levels up to: | |||
| 8.0000 % in the fragrance concentrate. | |||
| Maximised Survey-derived Daily Intakes (MSDI-EU): | 1.20 (μg/capita/day) | ||
| Maximised Survey-derived Daily Intakes (MSDI-USA): | 0.30 (μg/capita/day) | ||
| Threshold of Concern: | 1800 (μg/person/day) | ||
| Structure Class: | I | ||
| Use levels for FEMA GRAS flavoring substances on which the FEMA Expert Panel based its judgments that the substances are generally recognized as safe (GRAS). | |||
| The Expert Panel also publishes separate extensive reviews of scientific information on all FEMA GRAS flavoring substances and can be found at FEMA Flavor Ingredient Library | |||
| publication number: 3 | |||
| Click here to view publication 3 | |||
| average usual ppm | average maximum ppm | ||
| baked goods: | 1.50000 | 15.00000 | |
| beverages(nonalcoholic): | 0.12000 | 0.90000 | |
| beverages(alcoholic): | - | - | |
| breakfast cereal: | - | - | |
| cheese: | - | - | |
| chewing gum: | - | - | |
| condiments / relishes: | - | - | |
| confectionery froastings: | - | - | |
| egg products: | - | - | |
| fats / oils: | - | - | |
| fish products: | - | - | |
| frozen dairy: | 0.60000 | 6.00000 | |
| fruit ices: | 0.60000 | 6.00000 | |
| gelatins / puddings: | - | - | |
| granulated sugar: | - | - | |
| gravies: | - | - | |
| hard candy: | 1.50000 | 15.00000 | |
| imitation dairy: | - | - | |
| instant coffee / tea: | - | - | |
| jams / jellies: | - | - | |
| meat products: | - | - | |
| milk products: | - | - | |
| nut products: | - | - | |
| other grains: | - | - | |
| poultry: | - | - | |
| processed fruits: | - | - | |
| processed vegetables: | - | - | |
| reconstituted vegetables: | - | - | |
| seasonings / flavors: | - | - | |
| snack foods: | - | - | |
| soft candy: | - | - | |
| soups: | - | - | |
| sugar substitutes: | - | - | |
| sweet sauces: | - | - | |
Safety References:
| Flavor & Extract Manufacturers Association (FEMA) reference(s): | |
| The FEMA GRAS assessment of aromatic substituted secondary alcohols, ketones, and related esters used as flavor ingredients. View pdf | |
| European Food Safety Athority(EFSA): | Flavor usage levels; Subacute, Subchronic, Chronic and Carcinogenicity Studies; Developmental / Reproductive Toxicity Studies; Genotoxicity Studies... |
| European Food Safety Authority (EFSA) reference(s): | |
| Opinion of the Scientific Panel on food additives, flavourings, processing aids and materials in contact with food (AFC) related to Flavouring Group Evaluation 16 (FGE.16): Aromatic ketones from chemical group 21 (Commission Regulation (EC) No 1565/2000 of 18 July 2000) View page or View pdf | |
| Flavouring Group Evaluation 16, Revision 1 (FGE.16Rev1)[1]: Aromatic ketones from chemical group 21- Opinion of the Scientific Panel on Food Additives, Flavourings, Processing Aids and Materials in Contact with Food View page or View pdf | |
| Flavouring Group Evaluation 69, (FGE.69)[1] - Consideration of aromatic substituted secondary alcohols, ketones and related esters evaluated by JECFA (57th meeting) structurally related to aromatic ketones from chemical group 21 evaluated by EFSA in FGE.16 (2006) - Opinion of the Scientific Panel on Food Additives, Flavourings, Processing Aids and Materials in Contact with Food View page or View pdf | |
| Flavouring Group Evaluation 16, Revision 2 (FGE.16Rev2): Aromatic ketones from chemical group 21 View page or View pdf | |
| Scientific Opinion on Flavouring Group Evaluation 69, Revision 1 (FGE.69Rev1): consideration of aromatic substituted secondary alcohols, ketones and related esters evaluated by JECFA (57th meeting), structurally related to aromatic ketones from chemical group 21 evaluated by EFSA in FGE.16Rev2 View page or View pdf | |
| EPI System: | View |
| AIDS Citations: | Search |
| Cancer Citations: | Search |
| Toxicology Citations: | Search |
| EPA ACToR: | Toxicology Data |
| EPA Substance Registry Services (SRS): | Registry |
| Laboratory Chemical Safety Summary : | 61302 |
| National Institute of Allergy and Infectious Diseases: | Data |
| WGK Germany: | 3 |
| 4-phenylbutan-2-ol | |
| Chemidplus: | 0002344709 |
References:
| 4-phenylbutan-2-ol | |
| NIST Chemistry WebBook: | Search Inchi |
| Canada Domestic Sub. List: | 2344-70-9 |
| Pubchem (cid): | 61302 |
| Pubchem (sid): | 135017447 |
| Pherobase: | View |
Other Information:
| (IUPAC): | Atomic Weights of the Elements 2011 (pdf) |
| Videos: | The Periodic Table of Videos |
| tgsc: | Atomic Weights use for this web site |
| (IUPAC): | Periodic Table of the Elements |
| FDA Substances Added to Food (formerly EAFUS): | View |
| HMDB (The Human Metabolome Database): | HMDB31613 |
| FooDB: | FDB008249 |
| Export Tariff Code: | 2906.29.6000 |
| VCF-Online: | VCF Volatile Compounds in Food |
| ChemSpider: | View |
Potential Blenders and core components note
Potential Uses:
| floral | FR | |
| peony | FR | |
| rose | FR |
Occurrence (nature, food, other): note
| blackberry fruit Search PMC Picture |
Synonyms:
| benzenepropanol, a-methyl- | |
| butan-2-ol, 4-phenyl- | |
| 2- | butanol, 4-phenyl- |
| 2- | hydroxy-4-phenyl butane |
| 2- | hydroxy-4-phenylbutane |
| methyl 2-phenyl ethyl carbinol | |
| methyl 2-phenylethyl carbinol | |
| alpha- | methyl benzene propanol |
| methyl phenethyl carbinol | |
| methyl phenyl ethyl carbinol | |
| methyl-2-phenylethylcarbinol | |
| a- | methylbenzenepropanol |
| methylphenethylcarbinol | |
| phenethyl methyl carbinol | |
| 4- | phenyl butan-2-ol |
| phenyl ethyl methyl carbinol | |
| 1- | phenyl-3-butanol |
| 4- | phenylbutan-2-ol |
| phenylethyl methyl carbinol |





