Articles:
L-limonene
Notes:
Constit. of pine needle oil. Also present in ginger, nutmeg, pepper, mace, coriander and other herbs and spices
| Fragrance Demo Formulas | ||
| CAS Number: | 5989-54-8 | 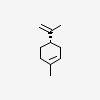 3D/inchi 3D/inchi
|
| Other(deleted CASRN): | 7721-11-1 | |
| ECHA EINECS - REACH Pre-Reg: | 227-815-6 | |
| FDA UNII: | 47MAJ1Y2NE | |
| Nikkaji Web: | J85.992C | |
| Beilstein Number: | 2323991 | |
| MDL: | MFCD00001558 | |
| CoE Number: | 491 | |
| XlogP3-AA: | 3.40 (est) | |
| Molecular Weight: | 136.23752000 | |
| Formula: | C10 H16 | |
| NMR Predictor: | Predict (works with chrome, Edge or firefox) | |
| EFSA/JECFA Comments: | With respect to specific gravity it is noted that limonene and l-limonene are submitted by different applicants. | |
Category: flavor and fragrance agents
US / EU / FDA / JECFA / FEMA / FLAVIS / Scholar / Patent Information:
| Google Scholar: | Search |
| Google Books: | Search |
| Google Scholar: with word "volatile" | Search |
| Google Scholar: with word "flavor" | Search |
| Google Scholar: with word "odor" | Search |
| Perfumer and Flavorist: | Search |
| Google Patents: | Search |
| US Patents: | Search |
| EU Patents: | Search |
| Pubchem Patents: | Search |
| PubMed: | Search |
| NCBI: | Search |
| DG SANTE Food Flavourings: | 01.046 L-limonene |
| FDA Mainterm (SATF): | 5989-54-8 ; L-LIMONENE |
| FDA Regulation: | |
| FDA PART 182 -- SUBSTANCES GENERALLY RECOGNIZED AS SAFE Subpart A--General Provisions Sec. 182.60 Synthetic flavoring substances and adjuvants. | |
Physical Properties:
| Appearance: | colorless clear liquid (est) |
| Assay: | 95.00 to 100.00 % |
| Food Chemicals Codex Listed: | Yes |
| Specific Gravity: | 0.84400 to 0.84800 @ 15.00 °C. |
| Pounds per Gallon - (est).: | 7.037 to 7.071 |
| Refractive Index: | 1.47100 to 1.47500 @ 20.00 °C. |
| Optical Rotation: | -118.0 to -108.0 |
| Boiling Point: | 176.00 to 177.00 °C. @ 760.00 mm Hg |
| Vapor Pressure: | 1.541000 mmHg @ 25.00 °C. (est) |
| Vapor Density: | 4.7 ( Air = 1 ) |
| Flash Point: | 127.00 °F. TCC ( 52.78 °C. ) |
| logP (o/w): | 4.380 |
| Soluble in: | |
| alcohol | |
| fixed oils | |
| water, 4.581 mg/L @ 25 °C (est) | |
| Insoluble in: | |
| water | |
Organoleptic Properties:
| Odor Type: terpenic | |
| Odor Strength: | medium |
| Substantivity: | 4 hour(s) at 100.00 % |
| terpenic pine herbal peppery | |
| Odor Description: at 100.00 %. | terpene pine herbal peppery Luebke, William tgsc, (1996) |
| Flavor Type: terpenic | |
| terpenic cilantro green juniper berry | |
| Taste Description: | terpenic cilantro green juniper berry Luebke, William tgsc, (1996) |
| Odor and/or flavor descriptions from others (if found). | |
| Indukern F&F | |
| L-LIMONENE | |
| Odor Description: | POWERFUL, TERPENIC |
Cosmetic Information:
| CosIng: | cosmetic data |
| Cosmetic Uses: |
perfuming agents |
Suppliers:
Safety Information:
| Preferred SDS: View | |
| European information : | |
| Most important hazard(s): | |
| Xi N - Irritant, Dangerous for the environment. | |
|
R 10 - Flammable. R 36/37/38 - Irritating to eyes, respiratory system, and skin. R 50/53 - Very toxic to aquatic organisms, may cause long-term adverse effects in the aquatic environment. R 65 - Harmful: may cause lung damage if swallowed. S 02 - Keep out of the reach of children. S 16 - Keep away from sources of ignition - No Smoking. S 36/37/39 - Wear suitable clothing, gloves and eye/face protection. S 60 - This material and its container must be disposed of as hazardous waste. S 61 - Avoid release to the environment. Refer to special instructions/safety data sheet. S 62 - If swallowed, do not induce vomiting: seek medical advice immediately and show this container or label. | |
| Hazards identification | |
| Classification of the substance or mixture | |
| GHS Classification in accordance with 29 CFR 1910 (OSHA HCS) | |
| Flammable liquids (Category 3), H226 Skin irritation (Category 2), H315 Skin sensitisation (Category 1), H317 Chronic aquatic toxicity (Category 1), H410 | |
| GHS Label elements, including precautionary statements | |
| Pictogram |    |
| Signal word | Warning |
| Hazard statement(s) | |
| H226 - Flammable liquid and vapour H315 - Causes skin irritation H317 - May cause an allergic skin reaction H410 - Very toxic to aquatic life with long lasting effects | |
| Precautionary statement(s) | |
| P210 - Keep away from heat/sparks/open flames/hot surfaces. — No smoking. P233 - Keep container tightly closed. P240 - Ground/bond container and receiving equipment. P241 - Use explosion-proof electrical/ventilating/lighting/…/equipment. P242 - Use only non-sparking tools. P243 - Take precautionary measures against static discharge. P261 - Avoid breathing dust/fume/gas/mist/vapours/spray. P264 - Wash skin thouroughly after handling. P272 - Contaminated work clothing should not be allowed out of the workplace. P273 - Avoid release to the environment. P280 - Wear protective gloves/protective clothing/eye protection/face protection. P303 + P361 + P353 - IF ON SKIN (or hair): Remove/Take off Immediately all contaminated clothing. Rinse SKIN with water/shower. P333 + P313 - IF SKIN irritation or rash occurs: Get medical advice/attention. P362 - Take off contaminated clothing and wash before reuse. P370 + P378 - In case of fire: Use dry sand, dry chemical or alcohol-resistant foam for extinction. P391 - Collect spillage. Hazardous to the aquatic environment P403 + P235 - Store in a well-ventilated place. Keep cool. P501 - Dispose of contents/ container to an approved waste disposal plant. | |
| Oral/Parenteral Toxicity: | |
|
oral-rat LD50 5000 mg/kg Dispose of contents/ container to an approved waste disposal plant. | |
| Dermal Toxicity: | |
|
skin-rabbit LD50 > 5000 mg/kg Dispose of contents/ container to an approved waste disposal plant. | |
| Inhalation Toxicity: | |
| Not determined | |
Safety in Use Information:
| Category: | flavor and fragrance agents | ||
| RIFM Fragrance Material Safety Assessment: Search | |||
| IFRA Code of Practice Notification of the 49th Amendment to the IFRA Code of Practice | |||
| IFRA Critical Effect: | Dermal sensitization | ||
| IFRA Other Specification: | < 20 mmoles / liter peroxides | ||
| IFRA fragrance material specification: | |||
| d-, l-and dl-Limonene and natural products containing substantial amounts of it, should only be used when the level of peroxides is kept to the lowest practical level, for instance by adding antioxidants at the time of production. Such products should have a peroxide value of less than 20 millimoles peroxides per liter, determined according to the FMA method, which can be downloaded from the IFRA website (see Analytical Methods). | |||
| IFRA: | View Standard | ||
| Recommendation for laevo-limonene usage levels up to: | |||
| 8.0000 % in the fragrance concentrate. | |||
| Maximised Survey-derived Daily Intakes (MSDI-EU): | 2100.00 (μg/capita/day) | ||
| Threshold of Concern: | 1800 (μg/person/day) | ||
| Structure Class: | I | ||
| Food categories according to Commission Regulation EC No. 1565/2000 (EC, 2000) in FGE.06 (EFSA, 2002a). According to the Industry the "normal" use is defined as the average of reported usages and "maximum use" is defined as the 95th percentile of reported usages (EFSA, 2002i). | |||
| Note: mg/kg = 0.001/1000 = 0.000001 = 1/1000000 = ppm. | |||
| average usage mg/kg | maximum usage mg/kg | ||
| Dairy products, excluding products of category 02.0 (01.0): | 3.00000 | 15.00000 | |
| Fats and oils, and fat emulsions (type water-in-oil) (02.0): | 2.00000 | 5.00000 | |
| Edible ices, including sherbet and sorbet (03.0): | 5.00000 | 10.00000 | |
| Processed fruit (04.1): | 1.00000 | 1.00000 | |
| Processed vegetables (incl. mushrooms & fungi, roots & tubers, pulses and legumes), and nuts & seeds (04.2): | - | - | |
| Confectionery (05.0): | 5.00000 | 15.00000 | |
| Chewing gum (05.0): | - | - | |
| Cereals and cereal products, incl. flours & starches from roots & tubers, pulses & legumes, excluding bakery (06.0): | 2.00000 | 10.00000 | |
| Bakery wares (07.0): | 5.00000 | 20.00000 | |
| Meat and meat products, including poultry and game (08.0): | 1.00000 | 5.00000 | |
| Fish and fish products, including molluscs, crustaceans and echinoderms (MCE) (09.0): | 1.00000 | 5.00000 | |
| Eggs and egg products (10.0): | 1.00000 | 5.00000 | |
| Sweeteners, including honey (11.0): | 1.00000 | 5.00000 | |
| Salts, spices, soups, sauces, salads, protein products, etc. (12.0): | 2.00000 | 15.00000 | |
| Foodstuffs intended for particular nutritional uses (13.0): | 3.00000 | 10.00000 | |
| Non-alcoholic ("soft") beverages, excl. dairy products (14.1): | 2.00000 | 10.00000 | |
| Alcoholic beverages, incl. alcohol-free and low-alcoholic counterparts (14.2): | 4.00000 | 10.00000 | |
| Ready-to-eat savouries (15.0): | 2.00000 | 5.00000 | |
| Composite foods (e.g. casseroles, meat pies, mincemeat) - foods that could not be placed in categories 01.0 - 15.0 (16.0): | 2.00000 | 5.00000 | |
Safety References:
| Flavor & Extract Manufacturers Association (FEMA) reference(s): | |
| The FEMA GRAS assessment of aliphatic and aromatic terpene hydrocarbons used as flavour ingredients. View pdf | |
| European Food Safety Athority(EFSA): | Flavor usage levels; Subacute, Subchronic, Chronic and Carcinogenicity Studies; Developmental / Reproductive Toxicity Studies; Genotoxicity Studies... |
| European Food Safety Authority (EFSA) reference(s): | |
| Scientific Opinion on Flavouring Group Evaluation 78, Revision 1 (FGE.78Rev1): Consideration of aliphatic and alicyclic and aromatic hydrocarbons evaluated by JECFA (63rd meeting) structurally related to aliphatic and aromatic hydrocarbons evaluated by EFSA in FGE.25Rev2 View page or View pdf | |
| Scientific Opinion on Flavouring Group Evaluation 25, Revision 2 (FGE.25Rev2): Aliphatic and aromatic hydrocarbons from chemical group 31 View page or View pdf | |
| Review of substances/agents that have direct beneficial effect on the environment: mode of action and assessment of efficacy View page or View pdf | |
| Scientific Opinion on the safety and efficacy of aliphatic and aromatic hydrocarbons (chemical group 31) when used as flavourings for all animal species View page or View pdf | |
| EPI System: | View |
| AIDS Citations: | Search |
| Cancer Citations: | Search |
| Toxicology Citations: | Search |
| EPA Substance Registry Services (TSCA): | 5989-54-8 |
| EPA ACToR: | Toxicology Data |
| EPA Substance Registry Services (SRS): | Registry |
| Laboratory Chemical Safety Summary : | 439250 |
| National Institute of Allergy and Infectious Diseases: | Data |
| SCCNFP: | opinion |
| WISER: | UN 2319 |
| WGK Germany: | 2 |
| (4S)-1-methyl-4-prop-1-en-2-ylcyclohexene | |
| Chemidplus: | 0005989548 |
| RTECS: | OS8350000 for cas# 5989-54-8 |
References:
| Leffingwell: | Chirality or Article |
| (4S)-1-methyl-4-prop-1-en-2-ylcyclohexene | |
| NIST Chemistry WebBook: | Search Inchi |
| Canada Domestic Sub. List: | 5989-54-8 |
| Pubchem (cid): | 439250 |
| Pubchem (sid): | 252080259 |
Other Information:
| (IUPAC): | Atomic Weights of the Elements 2011 (pdf) |
| Videos: | The Periodic Table of Videos |
| tgsc: | Atomic Weights use for this web site |
| (IUPAC): | Periodic Table of the Elements |
| FDA Substances Added to Food (formerly EAFUS): | View |
| CHEBI: | View |
| CHEMBL: | View |
| Golm Metabolome Database: | Search |
| KEGG (GenomeNet): | C06078 |
| HMDB (The Human Metabolome Database): | HMDB03375 |
| FooDB: | FDB013861 |
| Export Tariff Code: | 2902.19.0050 |
| Typical G.C. | |
| VCF-Online: | VCF Volatile Compounds in Food |
| ChemSpider: | View |
| Wikipedia: | View |
Potential Blenders and core components note
Potential Uses:
| acacia | FR | |
| apple green apple | FR | |
| bergamot | FR | |
| cajeput | FL/FR | |
| citrus | FR | |
| cologne | FR | |
| evergreen | FR | |
| fir balsam | FR | |
| fir needle oil replacer | FR | |
| green grass | FR | |
| herbal | FR | |
| lavender | FR | |
| mandarin | FR | |
| mint | FR | |
| neroli | FR | |
| orange blossom | FR | |
| peppermint | FR | |
| pine | FR | |
| pineapple | FR | |
| rain | FR | |
| spearmint | FR | |
| turpentine | FL/FR | |
| wormseed oil replacer | FR |
Occurrence (nature, food, other): note
| anise star anise Search Trop Picture | |
| anise star anise fruit Search Trop Picture | |
| cajuput Search Trop Picture | |
| caraway seed oil Search Trop Picture | |
| cardamom seed oil Search Trop Picture | |
| copal congo copal resin Search PMC Picture | |
| cornmint leaf Search Trop Picture | |
| cotton plant Search Trop Picture | |
| dill oil Search Trop Picture | |
| dill seed oil Search Trop Picture | |
| fennel seed Search Trop Picture | |
| horsemint Search Trop Picture | |
| pepper black pepper fruit Search Trop Picture | |
| pepper black pepper seed Search Trop Picture | |
| peppermint leaf Search Trop Picture | |
| pine needle oil Search PMC Picture | |
| sage Search Trop Picture | |
| spearmint Search Trop Picture | |
| spearmint leaf Search Trop Picture | |
| spearmint oil Search Picture | |
| spearmint plant Search Trop Picture | |
| tea tree oil australia @ trace% Data GC Search Trop Picture | |
| water mint leaf Search Trop Picture | |
| wormseed american Search Trop Picture |
Synonyms:
| cyclohexene, 1-methyl-4-(1-methylethenyl)-, (4S)- | |
| cyclohexene, 1-methyl-4-(1-methylethenyl)-, (S)- | |
| (-)- | limonene |
| (-)-(4S)- | limonene |
| (-)-(S)- | limonene |
| (4S)- | limonene |
| (4S)-(-)- | limonene |
| (S)- | limonene |
| (S)-(-)- | limonene |
| beta- | limonene |
| L- | limonene |
| L- | limonene - 65 |
| L- | limonene - 80 |
| laevo- | limonene (natural) |
| laevo- | limonene 75° |
| limonene L-65 | |
| limonene L-70 | |
| L- | limonene natural |
| laevo- | limonene natural |
| L- | limonene, natural |
| (S)-(-)-p- | mentha-1,8-diene |
| (S)-(-)-para- | mentha-1,8-diene |
| (S)-p- | mentha-1,8-diene |
| p- | mentha-1,8-diene, (S)-(-)- |
| (4S)-1- | methyl-4-(1-methyl ethenyl) cyclohexene |
| (S)-1- | methyl-4-(1-methyl ethenyl) cyclohexene |
| (S)-1- | methyl-4-(1-methyl vinyl) cyclohexene |
| (4S)-1- | methyl-4-(1-methylethenyl)cyclohexene |
| (S)-1- | methyl-4-(1-methylethenyl)cyclohexene |
| (S)-1- | methyl-4-(1-methylvinyl)cyclohexene |
| (4S)-1- | methyl-4-(prop-1-en-2-yl)cyclohex-1-ene |
| (4S)-1- | methyl-4-(prop-1-en-2-yl)cyclohexene |
| (4S)-1- | methyl-4-isopropenylcyclohex-1-ene |
| (4S)-1- | methyl-4-prop-1-en-2-ylcyclohexene |
| (S)-4-iso | propenyl-1-methyl cyclohexene |
| (4S)-4-iso | propenyl-1-methylcyclohexene |
| (S)-(-)-4-iso | propenyl-1-methylcyclohexene |



















