Articles:
dimethyl ketone
Notes:
a colorless liquid used as a solvent and an antiseptic. it is one of the ketone bodies produced during ketoacidosis. a colorless liquid used as a solvent and an antiseptic. it is one of the ketone bodies produced during ketoacidosis. Solvent used in food processing as a colour diluent, flavour ingredient, etc.
Acetone evaporates rapidly, even from water and soil. Once in the atmosphere, it is degraded by UV light with a 22-day half-life. Acetone dissipates slowly in soil, animals, or waterways since it is sometimes consumed by microorganisms; however, it is a significant issue with respect to groundwater contamination due to its high solubility in water. The LD50 of acetone for fish is 8.3 g/l of water (or about 0.8%) over 96 hours, and its environmental half-life is about 1 to 10 days. Acetone may pose a significant risk of oxygen depletion in aquatic systems due to the microbial activity consuming it.; Acetone is a good solvent for most plastics and synthetic fibres including those used in Nalgene bottles made of polystyrene, polycarbonate and some types of polypropylene.. It is ideal for thinning fiberglass resin, cleaning fiberglass tools and dissolving two-part epoxies and superglue before hardening. It is used as a volatile component of some paints and varnishes. As a heavy-duty degreaser, it is useful in the preparation of metal prior to painting; it also thins polyester resins, vinyl and adhesives.; Acetone is often the primary component in cleaning agents such as nail polish remover. Ethyl acetate, another organic solvent, is sometimes used as well. Acetone is a component of superglue remover and it easily removes residues from glass and porcelain.; Acetone is one of the ketone bodies produced during ketoacidosis. Acetone is not regarded as a waste product of metabolism. However, its physiological role in biochemical machinery is not clear. A model for the role of acetone metabolism is presented that orders the events occurring in acetonemia in sequence: in diabetic ketosis or starvation, ketone body production (b-hydroxy-butyrate, acetoacetate) provides fuel for vital organs (heart, brain . . .) raising the chance of survival of the metabolic catastrophe. However, when ketone body production exceeds the degrading capacity, the accumulating acetoacetic acid presents a new challenge to the pH regulatory system. Acetone production and its further degradation to C3 fragments fulfill two purposes: the maintenance of pH buffering capacity and provision of fuel for peripheral tissues. Since ketosis develops under serious metabolic circumstances, all the mechanisms that balance or moderate the effects of ketosis enhance the chance for survival. From this point of view, the theory that transportable C3 fragments can serve as additional nutrients is a novel view of acetone metabolism which introduces a new approach to the study of acetone degradation, especially in understanding its physiological function and the interrelationship between liver and peripheral tissues. (PMID 10580530). Acetone is typically derived from acetoacetate through the action of microbial acetoacetate decarboxylases found in gut microflora.; In chemistry, acetone is the simplest representative of the ketones. Acetone is a colorless, mobile, flammable liquid readily soluble in water, ethanol, ether, etc., and itself serves as an important solvent. ; Acetone is an irritant and inhalation may lead to hepatotoxic effects (causing liver damage).; Acetone is the organic compound with the formula OC(CH3)2. This colorless, mobile, flammable liquid is the simplest example of the ketones. Owing to the fact that acetone is miscible with water, and virtually all organic solvents, it serves as an important solvent in its own right, typically the solvent of choice for cleaning purposes in the laboratory. More than 3 billion kilograms are produced annually, mainly as a precursor to polymers. Familiar household uses of acetone are as the active ingredient in nail polish remover and as paint thinner and sanitary cleaner/ nail polish remover base. It is a common building block in organic chemistry. In addition to being manufactured, acetone also occurs naturally, even being biosynthesized in small amounts in the human body.; Bisphenol-A is a component of many polymers such as polycarbonates, polyurethanes, and epoxy resins.; In the laboratory, acetone is used as a polar aprotic solvent in a variety of organic reactions, such as SN2 reactions. The use of acetone solvent is also critical for the Jones oxidation. It is a common solvent for rinsing laboratory glassware because of its low cost, volatility, and ability to dissolve water. For similar reasons, acetone is also used as a drying agent. Acetone can be cooled with dry ice to -78 °C without freezing; acetone/dry ice baths are commonly used to conduct reactions at low temperatures. Acetone is fluorescent under ultraviolet light, and acetone vapor may be used as a fluorescent tracer in fluid flow experiments.; When oxidized, acetone forms acetone peroxide as a byproduct, which is a highly unstable compound. It may be formed accidentally, e.g. when waste hydrogen peroxide is poured into waste solvent containing acetone. Acetone peroxide is more than ten times as friction and shock sensitive as nitroglycerin. Due to its instability, it is rarely used, despite its easy chemical synthesis.
| CAS Number: | 67-64-1 | 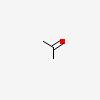 3D/inchi 3D/inchi
|
| ECHA EINECS - REACH Pre-Reg: | 200-662-2 | |
| FDA UNII: | 1364PS73AF | |
| Nikkaji Web: | J2.365E | |
| Beilstein Number: | 0635680 | |
| MDL: | MFCD00008765 | |
| CoE Number: | 737 | |
| XlogP3-AA: | -0.10 (est) | |
| Molecular Weight: | 58.08002000 | |
| Formula: | C3 H6 O | |
| BioActivity Summary: | listing | |
| NMR Predictor: | Predict (works with chrome or firefox) | |
Category: extraction solvents, flavoring agents
US / EU / FDA / JECFA / FEMA / FLAVIS / Scholar / Patent Information:
Physical Properties:
| Appearance: | colorless clear liquid (est) |
| Assay: | 99.00 to 100.00 % |
| Food Chemicals Codex Listed: | Yes |
| Specific Gravity: | 0.78800 to 0.79200 @ 25.00 °C. |
| Pounds per Gallon - (est).: | 6.557 to 6.590 |
| Refractive Index: | 1.34700 to 1.35900 @ 20.00 °C. |
| Melting Point: | -95.00 to -94.00 °C. @ 760.00 mm Hg |
| Boiling Point: | 56.00 to 57.00 °C. @ 760.00 mm Hg |
| Boiling Point: | 22.00 to 23.00 °C. @ 200.00 mm Hg |
| Vapor Pressure: | 232.000000 mmHg @ 25.00 °C. |
| Vapor Density: | 2.0 ( Air = 1 ) |
| Flash Point: | 1.00 °F. TCC ( -17.22 °C. ) |
| logP (o/w): | -0.160 |
| Shelf Life: | 24.00 month(s) or longer if stored properly. |
| Storage: | store in cool, dry place in tightly sealed containers, protected from heat and light. |
| Soluble in: | |
| alcohol | |
| water, 2.199e+005 mg/L @ 25 °C (est) | |
| water, 1.00E+06 mg/L @ 25 °C (exp) | |
| Similar Items: note | |
| acetone alcohol | |
Organoleptic Properties:
| Odor Type: solvent | |
| Odor Strength: | high , recommend smelling in a 1.00 % solution or less |
| Substantivity: | < 1 hour(s) at 100.00 % |
| solvent ethereal apple pear | |
| Odor Description: at 1.00 % in propylene glycol. | solvent ethereal apple pear |
| Odor and/or flavor descriptions from others (if found). | |
Cosmetic Information:
| CosIng: | cosmetic data |
| Cosmetic Uses: |
denaturants solvents |
Suppliers:
Safety Information:
| Preferred SDS: View | |
| European information : | |
| Most important hazard(s): | |
| Xi - Irritant | |
|
R 11 - Highly flammable. R 36 - Irritating to eyes. R 66 - Repeated exposure may cause skin dryness or cracking. R 67 - Vapours may cause frowsiness and dizziness. S 02 - Keep out of the reach of children. S 09 - Keep container in a well-ventilated place. S 16 - Keep away from sources of ignition - No Smoking. S 26 - In case of contact with eyes, rinse immediately with plenty of water and seek medical advice. | |
| Hazards identification | |
| Classification of the substance or mixture | |
| GHS Classification in accordance with 29 CFR 1910 (OSHA HCS) | |
| None found. | |
| GHS Label elements, including precautionary statements | |
| Pictogram | |
| Hazard statement(s) | |
| None found. | |
| Precautionary statement(s) | |
| None found. | |
| Oral/Parenteral Toxicity: | |
|
oral-rat LD50 [sex: M] 8452 mg/kg (Smyth et al., 1970) oral-rat LD50 8930 mg/kg (Smyth et al., 1969b) oral-rat LD50 9750 mg/kg (FDA, 1975a) oral-rat LD50 6800 mg/kg (Kimura et al., 1971a) oral-rat LD50 3465 mg/kg (Kohli et al., 1967) oral-mouse LD50 [sex: M] 5250 mg/kg (Tanii et al., 1986) oral-rabbit LD50 5300 mg/kg (Krasavage et al., 1982) intraperitoneal-dog LDLo 8000 mg/kg BEHAVIORAL: COMA GASTROINTESTINAL: ALTERATION IN GASTRIC SECRETION Archiv fuer Experimentelle Pathologie und Pharmakologie. Vol. 18, Pg. 218, 1884. oral-dog LDLo 8000 mg/kg MUSCULOSKELETAL: JOINTS BEHAVIORAL: COMA BEHAVIORAL: ATAXIA Archiv fuer Experimentelle Pathologie und Pharmakologie. Vol. 18, Pg. 218, 1884. oral-man TDLo 2857 mg/kg BEHAVIORAL: COMA KIDNEY, URETER, AND BLADDER: OTHER CHANGES "Toxicology of Drugs and Chemicals," Deichmann, W.B., New York, Academic Press, Inc., 1969Vol. -, Pg. 64, 1969. oral-man TDLo 2857 mg/kg BEHAVIORAL: COMA Diabetes. Vol. 15, Pg. 810, 1966. intraperitoneal-mouse LD50 1297 mg/kg Shell Chemical Company. Unpublished Report. Vol. -, Pg. 1, 1961. oral-mouse LD50 3000 mg/kg Pharmaceutical Chemistry Journal Vol. 14, Pg. 162, 1980. intravenous-mouse LDLo 4000 mg/kg FAO Nutrition Meetings Report Series. Vol. 48A, Pg. 86, 1970. oral-rabbit LD50 5340 mg/kg FAO Nutrition Meetings Report Series. Vol. 48A, Pg. 86, 1970. intravenous-rabbit LDLo 1576 mg/kg BEHAVIORAL: MUSCLE CONTRACTION OR SPASTICITY) LUNGS, THORAX, OR RESPIRATION: RESPIRATORY DEPRESSION Journal of Pharmacology and Experimental Therapeutics. Vol. 33, Pg. 175, 1928. intravenous-rat LD50 5500 mg/kg Raw Material Data Handbook, Vol.1: Organic Solvents, 1974. Vol. 1, Pg. 1, 1974. oral-rat LD50 5800 mg/kg BEHAVIORAL: ALTERED SLEEP TIME (INCLUDING CHANGE IN RIGHTING REFLEX) BEHAVIORAL: TREMOR Journal of Toxicology and Environmental Health. Vol. 15, Pg. 609, 1985. intraperitoneal-rat LDLo 500 mg/kg KIDNEY, URETER, AND BLADDER: RENAL FUNCTION TESTS DEPRESSED BEHAVIORAL: GENERAL ANESTHETIC BEHAVIORAL: MUSCLE WEAKNESS Journal of Pharmacy and Pharmacology. Vol. 11, Pg. 150, 1959. | |
| Dermal Toxicity: | |
|
skin-rabbit LDLo 20 ml/kg Union Carbide Data Sheet. Vol. 5/7/1970 skin-guinea pig LD50 > 9.40 ml/kg Toxicology and Applied Pharmacology. Vol. 7, Pg. 559, 1965. subcutaneous-dog LDLo 5000 mg/kg Archiv fuer Experimentelle Pathologie und Pharmakologie. Vol. 18, Pg. 218, 1884. skin-guinea pig LD50 > 9400 uL/kg Toxicology and Applied Pharmacology. Vol. 7, Pg. 559, 1965. subcutaneous-guinea pig LDLo 5000 mg/kg Archiv fuer Gewerbepathologie und Gewerbehygiene. Vol. 5, Pg. 1, 1933. | |
| Inhalation Toxicity: | |
|
inhalation-rat LC50 50100 mg/m3/8H American Industrial Hygiene Association Journal. Vol. 20, Pg. 364, 1959. inhalation-mouse LC50 44 gm/m3/4H Current Toxicology. Vol. 1, Pg. 47, 1993. inhalation-human TCLo 500 ppm SENSE ORGANS AND SPECIAL SENSES: CONJUNCTIVE IRRITATION: EYE LUNGS, THORAX, OR RESPIRATION: OTHER CHANGES SENSE ORGANS AND SPECIAL SENSES: OTHER CHANGES: OLFACTION Journal of Industrial Hygiene and Toxicology. Vol. 25, Pg. 282, 1943. inhalation-man TCLo 440 ug/m3/6M BRAIN AND COVERINGS: RECORDINGS FROM SPECIFIC AREAS OF CNS Gigiena i Sanitariya. For English translation, see HYSAAV. Vol. 42(8), Pg. 42, 1977. inhalation-man TCLo 10 mg/m3/6H Gigiena i Sanitariya. For English translation, see HYSAAV. Vol. 42(8), Pg. 42, 1977. inhalation-man TCLo 12000 ppm/4H BEHAVIORAL: MUSCLE WEAKNESS GASTROINTESTINAL: NAUSEA OR VOMITING Annals of Occupational Hygiene. Vol. 16, Pg. 73, 1973. inhalation-mouse LC50 44000 mg/m3/4H Current Toxicology. Vol. 1, Pg. 47, 1993. | |
Safety in Use Information:
| Category: | extraction solvents, flavoring agents | ||
| Recommendation for acetone usage levels up to: | |||
| not for fragrance use. | |||
| Maximised Survey-derived Daily Intakes (MSDI-EU): | 6100.00 (μg/capita/day) | ||
| Use levels for FEMA GRAS flavoring substances on which the FEMA Expert Panel based its judgments that the substances are generally recognized as safe (GRAS). | |||
| The Expert Panel also publishes separate extensive reviews of scientific information on all FEMA GRAS flavoring substances and can be found at FEMA Flavor Ingredient Library | |||
| publication number: 6 | |||
| Click here to view publication 6 | |||
| average usual ppm | average maximum ppm | ||
| baked goods: | - | 8.00000 | |
| beverages(nonalcoholic): | - | 5.00000 | |
| beverages(alcoholic): | - | - | |
| breakfast cereal: | - | - | |
| cheese: | - | - | |
| chewing gum: | - | - | |
| condiments / relishes: | - | - | |
| confectionery froastings: | - | - | |
| egg products: | - | - | |
| fats / oils: | - | - | |
| fish products: | - | - | |
| frozen dairy: | - | 5.00000 | |
| fruit ices: | - | 5.00000 | |
| gelatins / puddings: | - | 5.00000 | |
| granulated sugar: | - | - | |
| gravies: | - | - | |
| hard candy: | - | 8.00000 | |
| imitation dairy: | - | - | |
| instant coffee / tea: | - | - | |
| jams / jellies: | - | 5.00000 | |
| meat products: | - | - | |
| milk products: | - | - | |
| nut products: | - | - | |
| other grains: | - | - | |
| poultry: | - | - | |
| processed fruits: | - | - | |
| processed vegetables: | - | - | |
| reconstituted vegetables: | - | - | |
| seasonings / flavors: | - | - | |
| snack foods: | - | - | |
| soft candy: | - | - | |
| soups: | - | - | |
| sugar substitutes: | - | - | |
| sweet sauces: | - | - | |
Safety References:
| European Food Safety Athority(EFSA): | Flavor usage levels; Subacute, Subchronic, Chronic and Carcinogenicity Studies; Developmental / Reproductive Toxicity Studies; Genotoxicity Studies... |
| European Food Safety Authority (EFSA) reference(s): | |
| Opinion of the Scientific Panel on food additives, flavourings, processing aids and materials in contact with food (AFC) related to Flavouring Group Evaluation 7 (FGE.07): Saturated and unsaturated aliphatic secondary alcohols, ketones and esters of secondary alcohols and saturated linear or branched-chain carboxylic acids from chemical group 5 View page or View pdf | |
| Flavouring Group Evaluation 63 (FGE.63): Consideration of aliphatic secondary alcohols, ketones and related esters evaluated by JECFA (59th meeting) structurally related to saturated and unsaturated aliphatic secondary alcohols, ketones and esters of secondary alcohols and saturated linear or branched-chain carboxylic acids evaluated by EFSA in FGE.07 (2005) (Commission Regulation (EC) No 1565/2000 of 18 July 2000) View page or View pdf | |
| Flavouring Group Evaluation 7, Revision 1 (FGE.07Rev1): Saturated and unsaturated aliphatic secondary alcohols, ketones and esters of secondary alcohols and saturated linear or branched-chain carboxylic acids from chemical group 5 (Commission Regulation (EC) No 1565/2000 of 18 July 2000) - Scientific opinion of the Panel on Food Additives, Flavourings, Processing Aids and Materials in contact with Food (AFC) on a request from the Commission View page or View pdf | |
| Flavouring Group Evaluation 7, Revision 2 (FGE.07Rev2) : Saturated and unsaturated aliphatic secondary alcohols, ketones and esters of secondary alcohols and saturated linear or branched-chain carboxylic acids from chemical group 5 View page or View pdf | |
| EPI System: | View |
| EPA-Iris: | IRIS |
| ClinicalTrials.gov: | search |
| Daily Med: | search |
| NIOSH International Chemical Safety Cards: | search |
| NIOSH Pocket Guide: | search |
| Chemical Carcinogenesis Research Information System: | Search |
| AIDS Citations: | Search |
| Cancer Citations: | Search |
| Toxicology Citations: | Search |
| EPA GENetic TOXicology: | Search |
| EPA Substance Registry Services (TSCA): | 67-64-1 |
| EPA ACToR: | Toxicology Data |
| EPA Substance Registry Services (SRS): | Registry |
| Laboratory Chemical Safety Summary : | 180 |
| National Institute of Allergy and Infectious Diseases: | Data |
| WISER: | UN 1090 |
| WGK Germany: | 1 |
| propan-2-one | |
| Chemidplus: | 0000067641 |
| EPA/NOAA CAMEO: | hazardous materials |
| RTECS: | 67-64-1 |
References:
| propan-2-one | |
| NIST Chemistry WebBook: | Search Inchi |
| Canada Domestic Sub. List: | 67-64-1 |
| Pubchem (cid): | 180 |
| Pubchem (sid): | 134970724 |
| Pherobase: | View |
Other Information:
| (IUPAC): | Atomic Weights of the Elements 2011 (pdf) |
| Videos: | The Periodic Table of Videos |
| tgsc: | Atomic Weights use for this web site |
| (IUPAC): | Periodic Table of the Elements |
| FDA Substances Added to Food (formerly EAFUS): | View |
| FDA Indirect Additives used in Food Contact Substances: | View |
| CHEBI: | View |
| CHEMBL: | View |
| UM BBD: | Search |
| KEGG (GenomeNet): | C00207 |
| HMDB (The Human Metabolome Database): | HMDB01659 |
| FooDB: | FDB008301 |
| Export Tariff Code: | 2914.11.5000 |
| FDA Listing of Food Additive Status: | View |
| Typical G.C. | |
| VCF-Online: | VCF Volatile Compounds in Food |
| ChemSpider: | View |
| Wikipedia: | View |
Potential Blenders and core components note
Potential Uses:
| denaturants | ||
| solvents |
Occurrence (nature, food, other): note
| allspice plant Search Trop Picture | |
| apple fruit Search Trop Picture | |
| apple fruit juice Search Trop Picture | |
| apple plant Search Trop Picture | |
| bay laurel leaf Search Trop Picture | |
| beer Search PMC Picture | |
| bread white bread Search PMC Picture | |
| broccoli asparagus broccoli leaf Search Trop Picture | |
| broccoli asparagus broccoli root Search Trop Picture | |
| butter Search PMC Picture | |
| cabbage leaf Search Trop Picture | |
| carrot root Search Trop Picture | |
| cedarwood atlas Search Trop Picture | |
| cheese cheddar cheese Search PMC Picture | |
| cheese swiss cheese Search PMC Picture | |
| clove Search Trop Picture | |
| cocoa leaf Search Trop Picture | |
| coriander Search Trop Picture | |
| currant black currant fruit Search Trop Picture | |
| ginger oil Search Trop Picture | |
| ginger rhizome Search Trop Picture | |
| ginger rhizome oil Search Trop Picture | |
| goosefoot Search Trop Picture | |
| grape Search Trop Picture | |
| guava fruit Search Trop Picture | |
| lavandin oil abrialis @ 0.02% Data GC Search Trop Picture | |
| lavandin oil grosso @ trace% Data GC Search Trop Picture | |
| lavender Search Trop Picture | |
| lavender oil spike france @ 0.11% Data GC Search Trop Picture | |
| lemongrass plant Search Trop Picture | |
| mushroom giant puffball mushroom Search PMC Picture | |
| onion Search Trop Picture | |
| orange bitter orange Search Trop Picture | |
| paprika Search PMC Picture | |
| pear fruit Search Trop Picture | |
| pear plant Search Trop Picture | |
| pineapple fruit Search Trop Picture | |
| potato chip Search PMC Picture | |
| potato plant Search Trop Picture | |
| raspberry red raspberry fruit Search Trop Picture | |
| rice cooked rice Search PMC Picture | |
| rice plant Search Trop Picture | |
| soybean plant Search Trop Picture | |
| spearmint oil Search Trop Picture | |
| strawberry wild strawberry fruit Search Trop Picture | |
| tagete flower oil rwanda @ trace% Data GC Search Trop Picture | |
| tagete oil rwanda @ 0.63% Data GC Search Trop Picture | |
| tea leaf Search Trop Picture | |
| tomato fruit Search Trop Picture | |
| turpentine Search PMC Picture | |
| water mint leaf Search Trop Picture | |
| yogurt Search PMC Picture |
Synonyms:
| acetone 99%, (naturals) | |
| acetone FCC | |
| acetone min. 99%, natural | |
| acetone natural | |
| acetone, natural | |
| chevron acetone | |
| dimethyl formaldehyde | |
| dimethyl ketal | |
| dimethyl ketone | |
| dimethylformaldehyde | |
| dimethylketal | |
| ketone propane | |
| ketone, dimethyl | |
| methyl ketone | |
| propan-2-one | |
| 2-oxo | propane |
| beta-keto | propane |
| propanone | |
| 2- | propanone |
| pyroacetic acid | |
| pyroacetic ether |














