|
Category: flavor and fragrance agents
US / EU / FDA / JECFA / FEMA / FLAVIS / Scholar / Patent Information:
Physical Properties:
| Appearance: | amber solid (est) |
| Assay: | 98.00 to 100.00 % sum of isomers
|
| Food Chemicals Codex Listed: | No |
| Melting Point: | 105.00 to 107.00 °C. @ 760.00 mm Hg
|
| Boiling Point: | 218.00 to 220.00 °C. @ 19.00 mm Hg
|
| Flash Point: | 336.00 °F. TCC ( 169.10 °C. ) (est)
|
| logP (o/w): | 5.233 (est) |
| Soluble in: |
| | alcohol | | | water, 0.08174 mg/L @ 25 °C (est) |
Organoleptic Properties:
Cosmetic Information:
Suppliers:
Safety Information:
| Preferred SDS: View |
| European information : |
| Most important hazard(s): | | None - None found. |
S 02 - Keep out of the reach of children.
S 24/25 - Avoid contact with skin and eyes.
|
| |
| Hazards identification |
| |
| Classification of the substance or mixture |
| GHS Classification in accordance with 29 CFR 1910 (OSHA HCS) |
| None found. |
| GHS Label elements, including precautionary statements |
| |
| Pictogram | |
| |
| Hazard statement(s) |
| None found. |
| Precautionary statement(s) |
| None found. |
| Oral/Parenteral Toxicity: |
oral-rat LD50 > 5000 mg/kg
Food and Chemical Toxicology. Vol. 30, Pg. 115S, 1992.
|
| Dermal Toxicity: |
skin-rabbit LD50 > 5000 mg/kg
Food and Chemical Toxicology. Vol. 30, Pg. 115S, 1992.
|
| Inhalation Toxicity: |
|
Not determined
|
Safety in Use Information:
| Category: | flavor and fragrance agents |
| RIFM Fragrance Material Safety Assessment: Search |
| IFRA Code of Practice Notification of the 49th Amendment to the IFRA Code of Practice |
| IFRA Critical Effect: | Dermal sensitization |
| IFRA Purity Specification: | 98% minimum purity |
| IFRA fragrance material specification: | | | Sclareol used as a fragrance ingredient should have a minimum purity of 98%. |
| IFRA: | View Standard |
| maximum skin levels for fine fragrances: | | | 0.0200 % and are based on the assumption that the fragrance mixture is used at 20% in a consumer product (IFRA Use Level Survey). (IFRA, 2004)
|
| Recommendation for sclareol usage levels up to: | | | 4.0000 % in the fragrance concentrate.
|
| use level in formulae for use in cosmetics: | | | 0.0300 %
|
| Dermal Systemic Exposure in Cosmetic Products: | | | 0.0008 mg/kg/day (IFRA, 2004)
|
| |
| Maximised Survey-derived Daily Intakes (MSDI-EU): | 0.67 (μg/capita/day) |
| Modified Theoretical Added Maximum Daily Intake (mTAMDI): | 3900 (μg/person/day) |
| Threshold of Concern: | 1800 (μg/person/day) |
| Structure Class: | I |
| Use levels for FEMA GRAS flavoring substances on which the FEMA Expert Panel based its judgments that the substances are generally recognized as safe (GRAS). |
| The Expert Panel also publishes separate extensive reviews of scientific information on all FEMA GRAS flavoring substances and can be found at FEMA Flavor Ingredient Library |
| publication number: 24 |
| Click here to view publication 24 |
| | average usual ppm | average maximum ppm |
| baked goods: | 10.00000 | 50.00000 |
| beverages(nonalcoholic): | 5.00000 | 25.00000 |
| beverages(alcoholic): | 5.00000 | 25.00000 |
| breakfast cereal: | 5.00000 | 25.00000 |
| cheese: | 7.00000 | 35.00000 |
| chewing gum: | - | - |
| condiments / relishes: | 20.00000 | 100.00000 |
| confectionery froastings: | 10.00000 | 50.00000 |
| egg products: | - | - |
| fats / oils: | 5.00000 | 25.00000 |
| fish products: | 2.00000 | 10.00000 |
| frozen dairy: | 7.00000 | 35.00000 |
| fruit ices: | 10.00000 | 50.00000 |
| gelatins / puddings: | 10.00000 | 50.00000 |
| granulated sugar: | - | - |
| gravies: | 5.00000 | 25.00000 |
| hard candy: | 10.00000 | 50.00000 |
| imitation dairy: | 7.00000 | 35.00000 |
| instant coffee / tea: | 5.00000 | 25.00000 |
| jams / jellies: | 7.00000 | 35.00000 |
| meat products: | 2.00000 | 10.00000 |
| milk products: | 7.00000 | 35.00000 |
| nut products: | 5.00000 | 25.00000 |
| other grains: | 5.00000 | 25.00000 |
| poultry: | 2.00000 | 10.00000 |
| processed fruits: | 7.00000 | 35.00000 |
| processed vegetables: | 7.00000 | 35.00000 |
| reconstituted vegetables: | 7.00000 | 35.00000 |
| seasonings / flavors: | 5.00000 | 25.00000 |
| snack foods: | 10.00000 | 50.00000 |
| soft candy: | 10.00000 | 50.00000 |
| soups: | 5.00000 | 25.00000 |
| sugar substitutes: | - | - |
| sweet sauces: | 5.00000 | 25.00000 |
| |
| Food categories according to Commission Regulation EC No. 1565/2000 (EC, 2000) in FGE.06 (EFSA, 2002a). According to the Industry the "normal" use is defined as the average of reported usages and "maximum use" is defined as the 95th percentile of reported usages (EFSA, 2002i). |
| Note: mg/kg = 0.001/1000 = 0.000001 = 1/1000000 = ppm. |
| | average usage mg/kg | maximum usage mg/kg |
| Dairy products, excluding products of category 02.0 (01.0): | 7.00000 | 35.00000 |
| Fats and oils, and fat emulsions (type water-in-oil) (02.0): | 5.00000 | 25.00000 |
| Edible ices, including sherbet and sorbet (03.0): | 10.00000 | 50.00000 |
| Processed fruit (04.1): | 7.00000 | 35.00000 |
| Processed vegetables (incl. mushrooms & fungi, roots & tubers, pulses and legumes), and nuts & seeds (04.2): | - | - |
| Confectionery (05.0): | 10.00000 | 50.00000 |
| Chewing gum (05.0): | - | - |
| Cereals and cereal products, incl. flours & starches from roots & tubers, pulses & legumes, excluding bakery (06.0): | 5.00000 | 25.00000 |
| Bakery wares (07.0): | 10.00000 | 50.00000 |
| Meat and meat products, including poultry and game (08.0): | 2.00000 | 10.00000 |
| Fish and fish products, including molluscs, crustaceans and echinoderms (MCE) (09.0): | 2.00000 | 10.00000 |
| Eggs and egg products (10.0): | - | - |
| Sweeteners, including honey (11.0): | - | - |
| Salts, spices, soups, sauces, salads, protein products, etc. (12.0): | 5.00000 | 25.00000 |
| Foodstuffs intended for particular nutritional uses (13.0): | 10.00000 | 50.00000 |
| Non-alcoholic ("soft") beverages, excl. dairy products (14.1): | 5.00000 | 25.00000 |
| Alcoholic beverages, incl. alcohol-free and low-alcoholic counterparts (14.2): | 10.00000 | 50.00000 |
| Ready-to-eat savouries (15.0): | 20.00000 | 100.00000 |
| Composite foods (e.g. casseroles, meat pies, mincemeat) - foods that could not be placed in categories 01.0 - 15.0 (16.0): | 5.00000 | 25.00000 |
Safety References:
| European Food Safety Athority(EFSA): | Flavor usage levels; Subacute, Subchronic, Chronic and Carcinogenicity Studies; Developmental / Reproductive Toxicity Studies; Genotoxicity Studies... |
| European Food Safety Authority (EFSA) reference(s): |
Flavouring Group Evaluation 18, Revision 1 (FGE. 18 Rev1)[1] : Aliphatic, alicyclic and aromatic saturated and unsaturated tertiary alcohols, aromatic tertiary alcohols and their esters from chemical groups 6 and 8
View page or View pdf |
Scientific Opinion on Flavouring Group Evaluation 90 (FGE.90): Consideration of Aliphatic, acyclic and alicyclic terpenoid tertiary alcohols and structurally related substances evaluated by JECFA (68th meeting)FGE.18Rev1 (2009)
View page or View pdf |
Scientific Opinion on Flavouring Group Evaluation 18, Revision 2 (FGE.18Rev2): Aliphatic, alicyclic and aromatic saturated and unsaturated tertiary alcohols, aromatic tertiary alcohols and their esters from chemical groups 6 and 8.
View page or View pdf |
Scientific Opinion on Flavouring Group Evaluation 18, Revision 3 (FGE.18Rev3): Aliphatic, alicyclic and aromatic saturated and unsaturated tertiary alcohols, aromatic tertiary alcohols and their esters from chemical groups 6 and 8.
View page or View pdf |
| EPI System: | View |
| AIDS Citations: | Search |
| Cancer Citations: | Search |
| Toxicology Citations: | Search |
| EPA Substance Registry Services (TSCA): | 515-03-7 |
| EPA ACToR: | Toxicology Data |
| EPA Substance Registry Services (SRS): | Registry |
| Laboratory Chemical Safety Summary : | 73114 |
| National Institute of Allergy and Infectious Diseases: | Data |
| SCCNFP: | opinion |
| WGK Germany: | 2 |
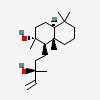
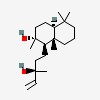
| | (1R,2R,8aS)-1-[(3R)-3-hydroxy-3-methylpent-4-enyl]-2,5,5,8a-tetramethyl-3,4,4a,6,7,8-hexahydro-1H-naphthalen-2-ol |
| Chemidplus: | 0000515037 |
| RTECS: | QK0301900 for cas# 515-03-7 |
| | (1R,2R,4aS,8aS)-1-[(3R)-3-hydroxy-3-methylpent-4-enyl]-2,5,5,8a-tetramethyl-3,4,4a,6,7,8-hexahydro-1H-naphthalen-2-ol |
References:
| | (1R,2R,8aS)-1-[(3R)-3-hydroxy-3-methylpent-4-enyl]-2,5,5,8a-tetramethyl-3,4,4a,6,7,8-hexahydro-1H-naphthalen-2-ol |
| NIST Chemistry WebBook: | Search Inchi |
| Canada Domestic Sub. List: | 515-03-7 |
| Pubchem (cid): | 73114 |
| Pubchem (sid): | 135029099 |
| | (1R,2R,4aS,8aS)-1-[(3R)-3-hydroxy-3-methylpent-4-enyl]-2,5,5,8a-tetramethyl-3,4,4a,6,7,8-hexahydro-1H-naphthalen-2-ol |
| NIST Chemistry WebBook: | Search Inchi |
| Canada Domestic Sub. List: | 515-03-7 |
| Pubchem (cid): | 163263 |
| Pubchem (sid): | 46237757 |
Other Information:
Potential Blenders and core components note
| |
| For Odor |
| amber |
| amber |
| (Z)- | abienol | FL/FR |
| | acetoxymethyl isolongifolene | FR |
| | amber acetate | FR |
| | amber butanol | FR |
| | amber carane | FR |
| | amber cyclohexanol | FR |
| | amber decane | FR |
| | amber furan | FR |
| | amber naphthofuran | FL/FR |
| | amber oxepin | FR |
| | amber specialty | FR |
| | amber spirolene | FR |
| | ambergris naphthol | FR |
| | ambermax 50 (Givaudan) | FR |
| | ambrette seed absolute | FL/FR |
| | ambrinol | FR |
| | ambroxan | FL/FR |
| | formoxymethyl isolongifolene | FR |
| | hydroxymethyl isolongifolene 50% in dpg | FR |
| | labdanum absolute | FL/FR |
| animal |
| | animal carbolactone | FR |
| balsamic |
| | amyris wood oil | FL/FR |
| | copaiba balsam oil | FL/FR |
| | copaifera reticulata extract | FL/FR |
| | fir carboxylate | FR |
| | mastic gum resin | FR |
| | methyl hydrogenated rosinate | FR |
| | myrrh oil | FL/FR |
| | opoponax resin (commiphora erythraea var. glabrescens engler) | FL/FR |
| | opoponax resinoid replacer | FR |
| | oriental specialty | FR |
| | peru balsam absolute | FL/FR |
| | styrax resin (liquidambar styraciflua) | FL/FR |
| | styrax resinoid (liquidambar styraciflua) | FL/FR |
| | styrax resinoid replacer | FR |
| camphoreous |
| beta-homo | cyclocitral | FL/FR |
| citrus |
| | citronella fragrance | FR |
| | citronella oil | FL/FR |
| | citronella oil java | FR |
| | grapefruit oil c.p. california | FL/FR |
| bitter | orange peel oil brazil | FL/FR |
| | valencene | FL/FR |
| dusty |
| | woody furan | FR |
| floral |
| 4-tert- | butyl cyclohexane carboxaldehyde | FR |
| | cananga oil | FL/FR |
| | cassie perfume base | FR |
| | decanal / methyl anthranilate schiff's base | FR |
| | heliotropin | FL/FR |
| (Z)-3- | hexen-1-yl salicylate | FL/FR |
| | orris pyridine 25% IPM | FR |
| | rose absolute (rosa centifolia) morocco | FL/FR |
| fruity |
| | balsam specialty | FR |
| | cyclohexyl crotonate | FR |
| green |
| | bicyclogermacrene | |
| iso | butyl benzyl carbinol | FL/FR |
| hay |
| | tobacco leaf absolute | FL/FR |
| herbal |
| | amber dioxepine | FR |
| | artemisia vestita wall. leaf oil | FR |
| (+)-alpha- | campholenic aldehyde | FL/FR |
| | clary acetate | FR |
| | clary octenone | FR |
| | clary propyl acetate | FR |
| | clary sage oil france | FL/FR |
| | clary sage oil replacer | FR |
| | clary sage oil russia | FL/FR |
| | clary sage resin america | FR |
| | romanal | FR |
| | rosemary oil | FL/FR |
| | rosemary oil CO2 extract | FL/FR |
| | rosemary oil spain | FL/FR |
| minty |
| | spearmint absolute | FL/FR |
| mossy |
| | moss fragrance | FR |
| | moss specialty | FR |
| | treemoss absolute | FR |
| | veramoss (IFF) | FR |
| musk |
| (Z)- | civet decenone | FL/FR |
| | cyclohexadecanone | FR |
| | juniper lactone | FL/FR |
| | musk fragrance | FR |
| | musk methyl ketone | FR |
| | musk specialty | FR |
| omega- | pentadecalactone | FL/FR |
| 6,6,10,10- | tetramethyl-5,7,8,9,10,10a-hexahydro-6H-6a,9-methanobenzo[H]quinazoline | |
| nutty |
| | pistachio fragrance | FR |
| oily |
| | mcp acetate | FR |
| powdery |
| alpha- | methyl ionone | FL/FR |
| | midnight passion fragrance | FR |
| resinous |
| | mastic absolute | FL/FR |
| rummy |
| | rum extract | FL/FR |
| spicy |
| | angelica seed absolute | FL/FR |
| iso | butyl angelate | FL/FR |
| terpenic |
| | angelica seed oil | FL/FR |
| tobacco |
| | honey absolute | FL/FR |
| | veltonal (Bedoukian) | FR |
| waxy |
| | tetradecanal | FL/FR |
| woody |
| | acetyl cedrene | FR |
| | amber carbinol | FR |
| | amber decatriene | FR |
| | amber dioxane | FR |
| | amber formate | FR |
| | amber pentadecane | FR |
| | amber woody specialty | FR |
| | ambrene acetal | FR |
| beta- | caryophyllene alcohol acetate | FL/FR |
| | cedarwood oil texas | FR |
| | cedarwood oil texas fractions | FR |
| alpha- | cedrene epoxide | FR |
| | cedrenyl acetate | FR |
| | cedrol methyl ether | FR |
| | cedryl acetate | FL/FR |
| | cedryl formate | FR |
| | cistus twig/leaf oil | FL/FR |
| | dihydro-beta-ionone | FL/FR |
| | dragons blood fragrance | FR |
| | frankincense resinoid replacer | FR |
| | georgywood | FR |
| | guaiacwood oil | FL/FR |
| (1R,4S,5S,9R)-1- | hydroxy-1,4,7,7,9-pentamethyl spiro(4.5)decan-2-one | |
| | hydroxyambran | FR |
| 2- | methoxy-4-vinyl phenol | FL/FR |
| | methyl methylene tricyclodecanol acetate | FR |
| (4aR,5R,7aS,9R)- | octahydro-2,2,5,8,8,9a-hexamethyl-4h-4a,9-methanoazuleno(5,6-d)-1,3-dioxole | FR |
| | patchouli woody amber fragrance | FR |
| | sandalwood oil | FL/FR |
| | sclarene | |
| | sclareolide | FL/FR |
| | tetramethyl-4-methylene-2-heptanol | FR |
| | timber propanol | FR |
| | tobacarol (IFF) | FR |
| | tobacco nonene | FR |
| | verdoxan | FR |
| | vetiver oil china | FL/FR |
| | vetiver specialty | FR |
| | vetiverol | FL/FR |
| | woody amber fragrance | FR |
| | woody amber specialty | FR |
| | woody carboxylate | FR |
| | woody cyclohexanone | FR |
| | woody dioxolane | FR |
| | woody epoxide | FR |
| | woody ether | FR |
| | woody nonane (ethoxy) | FR |
| | woody propanol | FR |
| |
| For Flavor |
| |
| No flavor group found for these |
| | bicyclogermacrene | |
| beta- | caryophyllene alcohol acetate | FL/FR |
| | elecampane root oil | FL |
| (1R,4S,5S,9R)-1- | hydroxy-1,4,7,7,9-pentamethyl spiro(4.5)decan-2-one | |
| | sclarene | |
| 6,6,10,10- | tetramethyl-5,7,8,9,10,10a-hexahydro-6H-6a,9-methanobenzo[H]quinazoline | |
| amber |
| | amber naphthofuran | FL/FR |
| | angelica seed oil | FL/FR |
| iso | butyl benzyl carbinol | FL/FR |
| | labdanum absolute | FL/FR |
| balsamic |
| | copaiba balsam oil | FL/FR |
| | copaifera reticulata extract | FL/FR |
| | myrrh oil | FL/FR |
| | opoponax resin (commiphora erythraea var. glabrescens engler) | FL/FR |
| | peru balsam absolute | FL/FR |
| | styrax resin (liquidambar styraciflua) | FL/FR |
| | styrax resinoid (liquidambar styraciflua) | FL/FR |
| cherry |
| | heliotropin | FL/FR |
| citrus |
| | citronella oil | FL/FR |
| | grapefruit oil c.p. california | FL/FR |
| | valencene | FL/FR |
| cooling |
| beta-homo | cyclocitral | FL/FR |
| fatty |
| | tetradecanal | FL/FR |
| floral |
| | cananga oil | FL/FR |
| | rose absolute (rosa centifolia) morocco | FL/FR |
| fruity |
| alpha- | methyl ionone | FL/FR |
| bitter | orange peel oil brazil | FL/FR |
| grassy |
| | tobacco leaf absolute | FL/FR |
| green |
| iso | butyl angelate | FL/FR |
| (+)-alpha- | campholenic aldehyde | FL/FR |
| (Z)-3- | hexen-1-yl salicylate | FL/FR |
| herbal |
| | clary sage oil france | FL/FR |
| | clary sage oil russia | FL/FR |
| | rosemary oil | FL/FR |
| | rosemary oil CO2 extract | FL/FR |
| | rosemary oil spain | FL/FR |
| honey |
| | honey absolute | FL/FR |
| minty |
| | spearmint absolute | FL/FR |
| musk |
| (Z)- | civet decenone | FL/FR |
| | juniper lactone | FL/FR |
| resinous |
| | mastic absolute | FL/FR |
| rummy |
| | rum extract | FL/FR |
| | rum flavor | FL |
| smoky |
| 2- | methoxy-4-vinyl phenol | FL/FR |
| spicy |
| | angelica seed absolute | FL/FR |
| | clove flavor | FL |
| vanilla |
| omega- | pentadecalactone | FL/FR |
| woody |
| (Z)- | abienol | FL/FR |
| | ambrette seed absolute | FL/FR |
| | ambroxan | FL/FR |
| | amyris wood oil | FL/FR |
| | cedryl acetate | FL/FR |
| | cistus twig/leaf oil | FL/FR |
| | dihydro-beta-ionone | FL/FR |
| | guaiacwood oil | FL/FR |
| | sandalwood oil | FL/FR |
| | sclareolide | FL/FR |
| | vetiver oil china | FL/FR |
| | vetiverol | FL/FR |
| |
Potential Uses:
Occurrence (nature, food, other): noteSynonyms:
| alpha- | ethenyl decahydro-2-hydroxy-a,2,5,5,8a-pentamethyl(1R-(1alpha(R*),2beta,4abeta,8H | | (1theta- (1alpha (theta),2beta, 4abeta,8aalpha))-alpha- | ethenyl decahydro-2-hydroxy-alpha,2,5,5,8a-pentamethyl-1-naphthalene propanol | | (1r,2r,8as)-1-[(3R)-3- | hydroxy-3-methylpent-4-en-1-yl]-2,5,5,8a-tetramethyldecahydronaphthalen-2-ol | | (1R,2R,8aS)-1-[(3R)-3- | hydroxy-3-methylpent-4-enyl]-2,5,5,8a-tetramethyl-3,4,4a,6,7,8-hexahydro-1H-naphthalen-2-ol | | (1R-(1alpha(R*), 2beta,4abeta, 8aalpha))-2- | hydroxy-alpha,2,5,5,8a-pentamethyl-alpha-vinyl decahydronaphthalene-1-propan-1-ol | | (13R)- | labd-14-ene-8,13-diol | | | labd-14-ene-8,13-diol, (13R)- | | 1- | naphthalenepropanol, a-ethenyldecahydro-2-hydroxy-a,2,5,5,8a-pentamethyl-, (aR,1R,2R,4aS,8aS)- | | (-)- | sclareol | | | sclareol natural |
Articles:
| PubMed: | Characterization of two genes for the biosynthesis of abietane-type diterpenes in rosemary (Rosmarinus officinalis) glandular trichomes. |
| PubMed: | [Engineering Saccharomyces cerevisiae for sclareol production]. |
| PubMed: | [Preface for special issue on synthetic biology (2013)]. |
| PubMed: | Sclareol reduces CD4+ CD25+ FoxP3+ Treg cells in a breast cancer model in vivo. |
| PubMed: | Hh signaling inhibitors from Vitex negundo; naturally occurring inhibitors of the GLI1-DNA complex. |
| PubMed: | Chemical composition and anticancer activity of essential oils of Mediterranean sage (Salvia officinalis L.) grown in different environmental conditions. |
| PubMed: | Extracellular localization of the diterpene sclareol in clary sage (Salvia sclarea L., Lamiaceae). |
| PubMed: | Bioactive constituents of Salvia chrysophylla Stapf. |
| PubMed: | Toward a biosynthetic route to sclareol and amber odorants. |
| PubMed: | Enzymatic biotransformation of terpenes as bioactive agents. |
| PubMed: | A diterpene synthase from the clary sage Salvia sclarea catalyzes the cyclization of geranylgeranyl diphosphate to (8R)-hydroxy-copalyl diphosphate. |
| PubMed: | Cancer cell spheroids as a model to evaluate chemotherapy protocols. |
| PubMed: | Discovery and functional characterization of two diterpene synthases for sclareol biosynthesis in Salvia sclarea (L.) and their relevance for perfume manufacture. |
| PubMed: | Identification of natural diterpenes that inhibit bacterial wilt disease in tobacco, tomato and Arabidopsis. |
| PubMed: | Sclareol exhibits anti-inflammatory activity in both lipopolysaccharide-stimulated macrophages and the λ-carrageenan-induced paw edema model. |
| PubMed: | Antimicrobial evaluation of diterpenes from Copaifera langsdorffii oleoresin against periodontal anaerobic bacteria. |
| PubMed: | First enantiospecific synthesis of marine sesquiterpene quinol akaol A. |
| PubMed: | Four potato (Solanum tuberosum) ABCG transporters and their expression in response to abiotic factors and Phytophthora infestans infection. |
| PubMed: | Synthesis of the scalarane sesterterpenoid 16-deacetoxy-12-epi-scalarafuranacetate. |
| PubMed: | The labdane diterpene sclareol (labd-14-ene-8, 13-diol) induces apoptosis in human tumor cell lines and suppression of tumor growth in vivo via a p53-independent mechanism of action. |
| PubMed: | Isolation, chemical, and biotransformation routes of labdane-type diterpenes. |
| PubMed: | Chemical composition and antimicrobial activity of the essential oils from Cleome spinosa. |
| PubMed: | Sclareol modulates the Treg intra-tumoral infiltrated cell and inhibits tumor growth in vivo. |
| PubMed: | Mitochondria-targeted liposomes improve the apoptotic and cytotoxic action of sclareol. |
| PubMed: | Hemisynthesis of two marine cheilanthane sesterterpenes from (-)-sclareol: first enantioselective synthesis of petrosaspongiolide R. |
| PubMed: | Fragrance material review on sclareol. |
| PubMed: | Diversity of essential oil glands of clary sage (Salvia sclarea L., Lamiaceae). |
| PubMed: | Microbial hydroxylation of sclareol by Rhizopus stolonifer. |
| PubMed: | Cell growth inhibitory action of an unusual labdane diterpene, 13-epi-sclareol in breast and uterine cancers in vitro. |
| PubMed: | Antibacterial and cytotoxic activity of the acetone extract of the flowers of Salvia sclarea and some natural products. |
| PubMed: | Diels-Alder cycloaddition approach to puupehenone-related metabolites: synthesis of the potent angiogenesis inhibitor 8-epipuupehedione. |
| PubMed: | Liposomes modify the subcellular distribution of sclareol uptake by HCT-116 cancer cell lines. |
| PubMed: | Sclareol induces apoptosis in human HCT116 colon cancer cells in vitro and suppression of HCT116 tumor growth in immunodeficient mice. |
| PubMed: | Structure elucidation and antibacterial activity of new fungal metabolites of sclareol. |
| PubMed: | Calorimetric study on the induction of interdigitated phase in hydrated DPPC bilayers by bioactive labdanes and correlation to their liposome stability: The role of chemical structure. |
| PubMed: | Hemisynthesis of new labdane derivatives from (-)-sclareol. |
| PubMed: | Synthesis of ent-thallusin. |
| PubMed: | A new route toward 7-Oxo-13-hydroxy-8,11,13-podocarpatrienes from labdane diterpenes. |
| PubMed: | Labd-14-ene-8,13-diol (sclareol) induces cell cycle arrest and apoptosis in human breast cancer cells and enhances the activity of anticancer drugs. |
| PubMed: | Cytotoxic and antitumor activity of liposome-incorporated sclareol against cancer cell lines and human colon cancer xenografts. |
| PubMed: | NpPDR1, a pleiotropic drug resistance-type ATP-binding cassette transporter from Nicotiana plumbaginifolia, plays a major role in plant pathogen defense. |
| PubMed: | First enantiospecific synthesis of the antitumor marine sponge metabolite (-)-15-oxopuupehenol from (-)-sclareol. |
| PubMed: | A comparative study of the effects of cholesterol and sclareol, a bioactive labdane type diterpene, on phospholipid bilayers. |
| PubMed: | Effect of 13-epi-sclareol on the bacterial respiratory chain. |
| PubMed: | Pathogen-responsive expression of a putative ATP-binding cassette transporter gene conferring resistance to the diterpenoid sclareol is regulated by multiple defense signaling pathways in Arabidopsis. |
| PubMed: | Identification of regulatory sequence elements within the transcription promoter region of NpABC1, a gene encoding a plant ABC transporter induced by diterpenes. |
| PubMed: | Antibacterial diterpenoids from Astragalus brachystachys. |
| PubMed: | The plant PDR family of ABC transporters. |
| PubMed: | Molecular basis of pimarane compounds as novel activators of large-conductance Ca(2+)-activated K(+) channel alpha-subunit. |
| PubMed: | The ABC transporter SpTUR2 confers resistance to the antifungal diterpene sclareol. |
| PubMed: | Chromatographic (GC-MS, HPLC) and virological evaluations of Salvia sclarea infected by BBWV-I. |
| PubMed: | A plant plasma membrane ATP binding cassette-type transporter is involved in antifungal terpenoid secretion. |
| PubMed: | Labdane type diterpenes down-regulate the expression of c-Myc protein, but not of Bcl-2, in human leukemia T-cells undergoing apoptosis. |
| PubMed: | Biotransformation of two cytotoxic terpenes, alpha-santonin and sclareol by Botrytis cinerea. |
| PubMed: | Potential nitrite scavengers as inhibitors of the formation of N-nitrosamines in solution and tobacco matrix systems. |
| PubMed: | Synthesis of 11,12-epoxydrim-8,12-en-11-ol, 11,12-diacetoxydrimane, and warburganal from (-)-sclareol. |
| PubMed: | Synthesis and antitumoral activities of marine ent-chromazonarol and related compounds. |
| PubMed: | Chemical analysis and antimicrobial activity of the resin Ladano, of its essential oil and of the isolated compounds. |
| PubMed: | The effect of sclareol on growth and cell cycle progression of human leukemic cell lines. |
| PubMed: | A concise synthesis and in vitro cytotoxicity of new labdane diterpenes. |
| PubMed: | A concise conversion of (-)-sclareol into (+)-coronarin E and (-)-7-epi-coronarin A |
| PubMed: | [Plant anatomical and phytochemical evaluation of Salvia species]. |
| PubMed: | Biorationals fromNicotiana protect cucumbers againstColletotrichum lagenarium (Pass.) ell. & halst disease development. |
| PubMed: | Terpenoids from Salvia sclarea. |
| PubMed: | Synthesis of manool-related labdane diterpenes as platelet aggregation inhibitors. |
| PubMed: | Identification of four biliary metabolites of the diterpene sclareol in the laboratory rat. |
| PubMed: | Leaf surface chemicals fromNicotiana affecting germination ofPeronospora tabacina (adam) sporangia. |
| PubMed: | Hydroxylation and glucoside conjugation in the microbial metabolism of the diterpene sclareol. |
| PubMed: | Microbial models of mammalian metabolism: fungal metabolism of the diterpene sclareol by Cunninghamella species. |
| PubMed: | Influences of diterpene sclareol glycol on some dopamine related behavior. |
| PubMed: | The effects of the diterpene sclareol glycol on seizures do not depend on central benzodiazepine receptors. |
| PubMed: | The response of diterpene sclareol glycol to acute hypoxia in mice. |
| PubMed: | [The effect of sclareol lactone and sclareol glycol on artificially induced lung metastases of Lewis lung carcinoma (a preliminary report)]. |
| PubMed: | Measures of anxiety, retention and stress in the rat following treatment with the diterpene sclareol glycol. |
| PubMed: | Diterpene sclareol glycol inhibits clonidine-induced aggressive responses in mice. |
| PubMed: | Effects of the diterpene sclareol glycol on convulsive seizures. |
| PubMed: | Effects of the diterpene sclareol glycol on body temperature in rats. |
| PubMed: | [Study of the effect of sclareol glycol diterpene on the release of adenohypophysial hormones prolactin, somatotropin and adenocorticotrophic hormone]. |
| PubMed: | [Study of the effect of sclareol glycol diterpene on the 3',5'-AMP level]. |
| PubMed: | Experimental study of essential oils from two varieties of cultivated thyme, sesquiterpene Germacron and diterpene Sclareol for cholagogic and choleretic activity. |
| PubMed: | [Effect of diterpene sklareol glycol on the conflict test and its correlation with diazepam]. |
| PubMed: | [Effects of sklareol-glycol on pentylenetetrazole-induced convulsions and its interaction with diazepam]. |
| PubMed: | BIOSYNTHESIS AND METABOLISM OF (14C)SCLAREOL. |
| PubMed: | Biosynthesis of sclareol, beta-sitosterol, and oleanolic acid from mevalonic acid-2-C-14. |
| PubMed: | Biosynthesis of 14C-sclareol and beta-sitosterol from 2-14C-mevalonic acid. |
| PubMed: | [Sclareol, its chemical constitution and use in the synthesis of aromatic principles of amber; a review]. |
| PubMed: | [Not Available]. |
|
 3D/inchi
3D/inchi
 3D/inchi
3D/inchi


















