Articles:
3-N-butylphthalide
Notes:
isolated from phenolic part of ligusticum wallichii franch. Potential nutriceutical
| CAS Number: | 6066-49-5 | 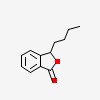 3D/inchi 3D/inchi
|
| Other(deleted CASRN): | 93133-67-6 | |
| ECHA EINECS - REACH Pre-Reg: | 228-000-8 | |
| FDA UNII: | 822Q956KGM | |
| Nikkaji Web: | J15.116E | |
| MDL: | MFCD01704513 | |
| CoE Number: | 10084 | |
| XlogP3: | 2.80 (est) | |
| Molecular Weight: | 190.24198000 | |
| Formula: | C12 H14 O2 | |
| BioActivity Summary: | listing | |
| NMR Predictor: | Predict (works with chrome or firefox) | |
| EFSA/JECFA Comments: | Racemate. | |
Category: flavor and fragrance agents
US / EU / FDA / JECFA / FEMA / FLAVIS / Scholar / Patent Information:
| Google Scholar: | Search |
| Google Books: | Search |
| Google Scholar: with word "volatile" | Search |
| Google Scholar: with word "flavor" | Search |
| Google Scholar: with word "odor" | Search |
| Perfumer and Flavorist: | Search |
| Google Patents: | Search |
| US Patents: | Search |
| EU Patents: | Search |
| Pubchem Patents: | Search |
| PubMed: | Search |
| NCBI: | Search |
| JECFA Food Flavoring: | 1169 3-N-butylphthalide |
| DG SANTE Food Flavourings: | 10.025 3-butylphthalide |
| FEMA Number: | 3334 3-N-butylphthalide |
| FDA: | No longer provide for the use of these seven synthetic flavoring substances |
| FDA Mainterm (SATF): | 6066-49-5 ; 3-N-BUTYLPHTHALIDE |
Physical Properties:
| Appearance: | colorless clear oily liquid (est) |
| Assay: | 97.00 to 100.00 % |
| Food Chemicals Codex Listed: | No |
| Specific Gravity: | 1.65100 to 1.06950 @ 25.00 °C. |
| Pounds per Gallon - (est).: | 13.738 to 8.899 |
| Refractive Index: | 1.51800 to 1.53200 @ 20.00 °C. |
| Boiling Point: | 177.00 to 178.00 °C. @ 15.00 mm Hg |
| Boiling Point: | 106.00 to 108.00 °C. @ 0.10 mm Hg |
| Acid Value: | 1.00 max. KOH/g |
| Vapor Pressure: | 0.001000 mmHg @ 25.00 °C. (est) |
| Flash Point: | 263.00 °F. TCC ( 128.33 °C. ) |
| logP (o/w): | 2.800 |
| Soluble in: | |
| alcohol | |
| water, 199.1 mg/L @ 25 °C (est) | |
| Insoluble in: | |
| water | |
Organoleptic Properties:
| Odor Type: herbal | |
| Odor Strength: | high , recommend smelling in a 10.00 % solution or less |
| herbal phenolic celery | |
| Odor Description: at 10.00 % in dipropylene glycol. | herbal phenolic celery |
| Odor and/or flavor descriptions from others (if found). | |
Cosmetic Information:
| CosIng: | cosmetic data |
| Cosmetic Uses: |
perfuming agents |
Suppliers:
| Alfa Biotechnology |
| For experimental / research use only. |
| Butylphthalide 98% |
| Axsyn |
| For experimental / research use only. |
| 3-Butylphthalide |
| BOC Sciences |
| For experimental / research use only. |
| 3-Butylphthalide >98% |
| Parchem |
| 3-butyl phthalide |
| Santa Cruz Biotechnology |
| For experimental / research use only. |
| 3-Butylphthalide ≥98% |
| Soda Aromatic |
| 3-Butyl Phthalide |
Safety Information:
| Preferred SDS: View | |
| Hazards identification | |
| Classification of the substance or mixture | |
| GHS Classification in accordance with 29 CFR 1910 (OSHA HCS) | |
| None found. | |
| GHS Label elements, including precautionary statements | |
| Pictogram | |
| Hazard statement(s) | |
| None found. | |
| Precautionary statement(s) | |
| None found. | |
| Human Experience: | |
| 2 % solution: no irritation or sensitization. | |
| Oral/Parenteral Toxicity: | |
|
oral-rat LD50 2450 mg/kg (Moreno, 1976ab) oral-mouse LD50 1850 mg/kg (Pellmont, 1970) oral-rat LD50 2450 mg/kg Food and Cosmetics Toxicology. Vol. 17, Pg. 251, 1979. | |
| Dermal Toxicity: | |
| Not determined | |
| Inhalation Toxicity: | |
| Not determined | |
Safety in Use Information:
| Category: | flavor and fragrance agents | ||
| IFRA Code of Practice Notification of the 49th Amendment to the IFRA Code of Practice | |||
| Recommendation for 3-butyl phthalide usage levels up to: | |||
| 2.0000 % in the fragrance concentrate. | |||
| Maximised Survey-derived Daily Intakes (MSDI-EU): | 0.49 (μg/capita/day) | ||
| Maximised Survey-derived Daily Intakes (MSDI-USA): | 0.40 (μg/capita/day) | ||
| Structure Class: | III | ||
| Use levels for FEMA GRAS flavoring substances on which the FEMA Expert Panel based its judgments that the substances are generally recognized as safe (GRAS). | |||
| The Expert Panel also publishes separate extensive reviews of scientific information on all FEMA GRAS flavoring substances and can be found at FEMA Flavor Ingredient Library | |||
| publication number: 6 | |||
| Click here to view publication 6 | |||
| average usual ppm | average maximum ppm | ||
| baked goods: | - | - | |
| beverages(nonalcoholic): | - | - | |
| beverages(alcoholic): | - | 0.50000 | |
| breakfast cereal: | - | - | |
| cheese: | - | - | |
| chewing gum: | - | - | |
| condiments / relishes: | - | 2.00000 | |
| confectionery froastings: | - | - | |
| egg products: | - | - | |
| fats / oils: | - | - | |
| fish products: | - | - | |
| frozen dairy: | - | - | |
| fruit ices: | - | - | |
| gelatins / puddings: | - | 2.00000 | |
| granulated sugar: | - | - | |
| gravies: | - | 1.00000 | |
| hard candy: | - | - | |
| imitation dairy: | - | - | |
| instant coffee / tea: | - | - | |
| jams / jellies: | - | 2.00000 | |
| meat products: | - | 1.00000 | |
| milk products: | - | - | |
| nut products: | - | - | |
| other grains: | - | - | |
| poultry: | - | - | |
| processed fruits: | - | - | |
| processed vegetables: | - | - | |
| reconstituted vegetables: | - | - | |
| seasonings / flavors: | - | - | |
| snack foods: | - | - | |
| soft candy: | - | - | |
| soups: | - | 1.00000 | |
| sugar substitutes: | - | - | |
| sweet sauces: | - | - | |
Safety References:
| Flavor & Extract Manufacturers Association (FEMA) reference(s): | |
| The FEMA GRAS assessment of lactones used as flavor ingredients. View pdf | |
| European Food Safety Athority(EFSA): | Flavor usage levels; Subacute, Subchronic, Chronic and Carcinogenicity Studies; Developmental / Reproductive Toxicity Studies; Genotoxicity Studies... |
| European Food Safety Authority (EFSA) reference(s): | |
| Flavouring Group Evaluation 27 (FGE.27): One aromatic lactone from chemical group 11[1] - Scientific Opinion of the Scientific Panel on Food Additives, Flavourings, Processing Aids and Materials in Contact with Food View page or View pdf | |
| Flavouring Group Evaluation 80 (FGE.80) - Consideration of alicyclic, alicyclic-fused and aromatic-fused ring lactones evaluated by JECFA (61st meeting) structurally related to a aromatic lactone evaluated by EFSA in FGE.27 (2008) View page or View pdf | |
| Flavouring Group Evaluation 80, Revision 1 (FGE.80Rev1): Consideration of alicyclic, alicyclic-fused and aromatic-fused ring lactones evaluated by JECFA (61st meeting) structurally related to a aromatic lactone evaluated by EFSA in FGE.27 (2008) View page or View pdf | |
| EPI System: | View |
| ClinicalTrials.gov: | search |
| Chemical Carcinogenesis Research Information System: | Search |
| Cancer Citations: | Search |
| Toxicology Citations: | Search |
| EPA Substance Registry Services (TSCA): | 6066-49-5 |
| EPA ACToR: | Toxicology Data |
| EPA Substance Registry Services (SRS): | Registry |
| Laboratory Chemical Safety Summary : | 61361 |
| National Institute of Allergy and Infectious Diseases: | Data |
| WGK Germany: | 3 |
| 3-butyl-3H-2-benzofuran-1-one | |
| Chemidplus: | 0006066495 |
References:
| Leffingwell: | Chirality or Article |
| 3-butyl-3H-2-benzofuran-1-one | |
| NIST Chemistry WebBook: | Search Inchi |
| Canada Domestic Sub. List: | 6066-49-5 |
| Pubchem (cid): | 61361 |
| Pubchem (sid): | 135018318 |
Other Information:
| (IUPAC): | Atomic Weights of the Elements 2011 (pdf) |
| Videos: | The Periodic Table of Videos |
| tgsc: | Atomic Weights use for this web site |
| (IUPAC): | Periodic Table of the Elements |
| FDA Substances Added to Food (formerly EAFUS): | View |
| CHEMBL: | View |
| KEGG (GenomeNet): | C17854 |
| HMDB (The Human Metabolome Database): | HMDB32064 |
| FooDB: | FDB011862 |
| Typical G.C. | |
| VCF-Online: | VCF Volatile Compounds in Food |
| ChemSpider: | View |
| Wikipedia: | View |
| EFSA Update of results on the monitoring of furan levels in food: | Read Report |
| EFSA Previous report: Results on the monitoring of furan levels in food: | Read Report |
| EFSA Report of the CONTAM Panel on provisional findings on furan in food: | Read Report |
Potential Blenders and core components note
Potential Uses:
| celery | FR | |
| herbal | FR | |
| spice | FR | |
| woody | FR |
Occurrence (nature, food, other): note
| carrot seed oil @ 0.27% Data GC Search Trop Picture | |
| celery leaf oil Search Trop Picture | |
| celery leaf oil @ 6.20% Data GC Search Trop Picture | |
| celery oil Search Trop Picture | |
| celery root Search Trop Picture | |
| celery seed Search Trop Picture | |
| celery seed oil Search Trop Picture | |
| celery seed oil CO2 extract india @ 5.49% Data GC Search Trop Picture | |
| celery seed oil egypt @ 0.38% Data GC Search Trop Picture | |
| celery seed oil india @ 2.68% Data GC Search Trop Picture | |
| dill root Search Trop Picture | |
| lovage root Search Trop Picture | |
| parsley leaf oil @ 0.78% Data GC Search Trop Picture | |
| parsley seed Search Trop Picture |
Synonyms:
| 3-N- | butyl phthalide |
| 3- | butyl-1(3H)-isobenzofuranone |
| 3- | butyl-2-benzofuran-1(3H)-one |
| 3- | butyl-3H-2-benzofuran-1-one |
| 3- | butyl-phthalide |
| 3- | butylisobenzofuran-1(3H)-one |
| 3- | butylphthalide |
| 3-N- | butylphthalide |
| phthalide, 3-butyl- |

