Articles:
pulegone
Notes:
Occurs in oils of Mentha spp., Hedeoma pulegioides and many other essential oils. Fragrance and flavour ingredient
| CAS Number: | 89-82-7 | 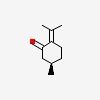 3D/inchi 3D/inchi
|
| Other(deleted CASRN): | 90449-51-7 | |
| ECHA EINECS - REACH Pre-Reg: | 201-943-2 | |
| FDA UNII: | 4LF2673R3G | |
| Nikkaji Web: | J3.216F | |
| Beilstein Number: | 2040703 | |
| MDL: | MFCD00063000 | |
| CoE Number: | 2050 | |
| XlogP3-AA: | 2.80 (est) | |
| Molecular Weight: | 152.23672000 | |
| Formula: | C10 H16 O | |
| BioActivity Summary: | listing | |
| NMR Predictor: | Predict (works with chrome, Edge or firefox) | |
Category: fragrance agents
US / EU / FDA / JECFA / FEMA / FLAVIS / Scholar / Patent Information:
| Google Scholar: | Search |
| Google Books: | Search |
| Google Scholar: with word "volatile" | Search |
| Google Scholar: with word "flavor" | Search |
| Google Scholar: with word "odor" | Search |
| Perfumer and Flavorist: | Search |
| Google Patents: | Search |
| US Patents: | Search |
| EU Patents: | Search |
| Pubchem Patents: | Search |
| PubMed: | Search |
| NCBI: | Search |
| JECFA Food Flavoring: | 753 pulegone |
| FEMA Number: | 2963-VOID pulegone |
| FDA: | No longer provide for the use of these seven synthetic flavoring substances |
| FDA Mainterm (SATF): | 89-82-7 ; PULEGONE |
| FDA Regulation: | |
| FDA PART 172 -- FOOD ADDITIVES PERMITTED FOR DIRECT ADDITION TO FOOD FOR HUMAN CONSUMPTION Subpart F--Flavoring Agents and Related Substances Sec. 172.515 Synthetic flavoring substances and adjuvants. | |
Physical Properties:
| Appearance: | colorless clear oily liquid (est) |
| Assay: | 85.00 to 100.00 % |
| Food Chemicals Codex Listed: | No |
| Boiling Point: | 220.00 to 222.00 °C. @ 760.00 mm Hg |
| Boiling Point: | 132.00 to 133.00 °C. @ 50.00 mm Hg |
| Vapor Pressure: | 0.093000 mmHg @ 25.00 °C. (est) |
| Flash Point: | 180.00 °F. TCC ( 82.22 °C. ) |
| logP (o/w): | 3.080 |
| Soluble in: | |
| alcohol | |
| water, 173.7 mg/L @ 25 °C (est) | |
| Insoluble in: | |
| water | |
Organoleptic Properties:
| Odor Type: minty | |
| Odor Strength: | medium |
| Substantivity: | 8 hour(s) at 100.00 % |
| peppermint camphoreous fresh herbal buchu | |
| Odor Description: at 100.00 %. | peppermint camphor fresh herbal buchu Luebke, William tgsc, (1985) |
| minty sulfurous sweet metallic buchu | |
| Odor Description: | Minty, sulfuraceous, sweet with metallic buchu nuances Mosciano, Gerard P&F 16, No. 1, 31, (1991) |
| Flavor Type: minty | |
| minty sulfurous fruity currant black currant raspberry fresh green leafy | |
| Taste Description: at 20.00 ppm. | Minty sulfuraceous, fruity blackcurrant and raspberry, with fresh green leafy nuances Mosciano, Gerard P&F 16, No. 1, 31, (1991) |
| Odor and/or flavor descriptions from others (if found). | |
Cosmetic Information:
| CosIng: | cosmetic data |
| Cosmetic Uses: |
perfuming agents |
Suppliers:
Safety Information:
| Preferred SDS: View | |
| European information : | |
| Most important hazard(s): | |
| Xn - Harmful. | |
|
R 22 - Harmful if swallowed. R 36/38 - Irritating to skin and eyes. S 02 - Keep out of the reach of children. S 20/21 - When using do not eat, drink or smoke. S 24/25 - Avoid contact with skin and eyes. S 26 - In case of contact with eyes, rinse immediately with plenty of water and seek medical advice. S 37/39 - Wear suitable gloves and eye/face protection. | |
| Hazards identification | |
| Classification of the substance or mixture | |
| GHS Classification in accordance with 29 CFR 1910 (OSHA HCS) | |
| None found. | |
| GHS Label elements, including precautionary statements | |
| Pictogram | |
| Hazard statement(s) | |
| None found. | |
| Precautionary statement(s) | |
| None found. | |
| Human Experience: | |
| 10 % solution: no irritation or sensitization. | |
| Oral/Parenteral Toxicity: | |
|
intravenous-dog LDLo 330 mg/kg VASCULAR: BP LOWERING NOT CHARACTERIZED IN AUTONOMIC SECTION CARDIAC: CHANGE IN RATE Comptes Rendus Hebdomadaires des Seances, Academie des Sciences. Vol. 236, Pg. 633, 1953. | |
| Dermal Toxicity: | |
| Not determined | |
| Inhalation Toxicity: | |
| Not determined | |
Safety in Use Information:
| Category: | fragrance agents | ||
| RIFM Fragrance Material Safety Assessment: Search | |||
| IFRA Code of Practice Notification of the 49th Amendment to the IFRA Code of Practice | |||
| Recommendation for (R)-(+)-pulegone usage levels up to: | |||
| 5.0000 % in the fragrance concentrate. | |||
| Use levels for FEMA GRAS flavoring substances on which the FEMA Expert Panel based its judgments that the substances are generally recognized as safe (GRAS). | |||
| The Expert Panel also publishes separate extensive reviews of scientific information on all FEMA GRAS flavoring substances and can be found at FEMA Flavor Ingredient Library | |||
| publication number: 3 | |||
| Click here to view publication 3 | |||
| average usual ppm | average maximum ppm | ||
| baked goods: | 24.00000 | 25.00000 | |
| beverages(nonalcoholic): | 5.00000 | 8.00000 | |
| beverages(alcoholic): | - | - | |
| breakfast cereal: | - | - | |
| cheese: | - | - | |
| chewing gum: | - | - | |
| condiments / relishes: | - | - | |
| confectionery froastings: | - | - | |
| egg products: | - | - | |
| fats / oils: | - | - | |
| fish products: | - | - | |
| frozen dairy: | 5.00000 | 32.00000 | |
| fruit ices: | 5.00000 | 32.00000 | |
| gelatins / puddings: | - | - | |
| granulated sugar: | - | - | |
| gravies: | - | - | |
| hard candy: | - | 17.00000 | |
| imitation dairy: | - | - | |
| instant coffee / tea: | - | - | |
| jams / jellies: | - | - | |
| meat products: | - | - | |
| milk products: | - | - | |
| nut products: | - | - | |
| other grains: | - | - | |
| poultry: | - | - | |
| processed fruits: | - | - | |
| processed vegetables: | - | - | |
| reconstituted vegetables: | - | - | |
| seasonings / flavors: | - | - | |
| snack foods: | - | - | |
| soft candy: | - | - | |
| soups: | - | - | |
| sugar substitutes: | - | - | |
| sweet sauces: | - | - | |
Safety References:
| Flavor & Extract Manufacturers Association (FEMA) reference(s): | |
| The FEMA GRAS assessment of alicyclic substances used as flavor ingredients. View pdf | |
| European Food Safety Authority (EFSA) reference(s): | |
| Pulegone and Menthofuran in flavourings - Opinion of the Scientific Panel on Food Additives, Flavourings, Processing Aids and Materials in contact with Food (AFC) View page or View pdf | |
| Flavouring Group Evaluation 57 (FGE.57)[1]: Consideration of two structurally related pulegone metabolites and one ester thereof evaluated by JECFA (55th meeting) View page or View pdf | |
| Scientific Opinion on Flavouring Group Evaluation 213, Revision 2 (FGE.213Rev2): Consideration of genotoxic potential for a,▀-unsaturated alicyclic ketones and precursors from chemical subgroup 2.7 of FGE.19 View page or View pdf | |
| EPI System: | View |
| Chemical Carcinogenesis Research Information System: | Search |
| AIDS Citations: | Search |
| Cancer Citations: | Search |
| Toxicology Citations: | Search |
| EPA Substance Registry Services (TSCA): | 89-82-7 |
| EPA ACToR: | Toxicology Data |
| EPA Substance Registry Services (SRS): | Registry |
| Laboratory Chemical Safety Summary : | 442495 |
| National Institute of Allergy and Infectious Diseases: | Data |
| WGK Germany: | 3 |
| (5R)-5-methyl-2-propan-2-ylidenecyclohexan-1-one | |
| Chemidplus: | 0000089827 |
| RTECS: | OT0261000 for cas# 89-82-7 |
References:
| (5R)-5-methyl-2-propan-2-ylidenecyclohexan-1-one | |
| NIST Chemistry WebBook: | Search Inchi |
| Canada Domestic Sub. List: | 89-82-7 |
| Pubchem (cid): | 442495 |
| Pubchem (sid): | 134972995 |
| Pherobase: | View |
Other Information:
| (IUPAC): | Atomic Weights of the Elements 2011 (pdf) |
| Videos: | The Periodic Table of Videos |
| tgsc: | Atomic Weights use for this web site |
| (IUPAC): | Periodic Table of the Elements |
| FDA Substances Added to Food (formerly EAFUS): | View |
| CHEBI: | View |
| CHEMBL: | View |
| KEGG (GenomeNet): | C09893 |
| HMDB (The Human Metabolome Database): | HMDB35604 |
| FooDB: | FDB014298 |
| Export Tariff Code: | 2914.29.5000 |
| Typical G.C. | |
| VCF-Online: | VCF Volatile Compounds in Food |
| ChemSpider: | View |
| Wikipedia: | View |
Potential Blenders and core components note
Potential Uses:
| currant black currant | FR | |
| grape | FR | |
| herbal | FR | |
| mint | FR | |
| peach | FR | |
| pennyroyal | FL/FR | |
| peppermint | FR | |
| raspberry | FR | |
| strawberry | FR | |
| tropical | FL | |
| woody | FR |
Occurrence (nature, food, other): note
| agathosma crenulata oil @ 53.75% Data GC Search Trop Picture | |
| bergamot plant wild Search Trop Picture | |
| blackberry fruit Search PMC Picture | |
| blueberry plant Search Trop Picture | |
| buchu leaf oil @ 0.94% Data GC Search Trop Picture | |
| calamintha nepeta (l.) savi subsp. glandulosa oil greece @ 41.00% Data GC Search Trop Picture | |
| cayenne fruit Search Trop Picture | |
| chamomile roman chamomile Search Trop Picture | |
| cornmint oil india @ 0.13% Data GC Search Trop Picture | |
| cornmint plant Search Trop Picture | |
| currant black currant bud Search Trop Picture | |
| currant black currant fruit Search Trop Picture | |
| horsemint shoot Search Trop Picture | |
| lemon balm Search Trop Picture | |
| lemongrass oil morocco @ 0.90% Data GC Search Trop Picture | |
| mint Search Trop Picture | |
| origanum Search PMC Picture | |
| pennyroyal Search Trop Picture | |
| pennyroyal oil @ 86.70% Data GC Search Trop Picture | |
| pennyroyal oil cuba @ 25.14% Data GC Search Trop Picture | |
| pennyroyal oil uruguay @ 26.8-41.10% Data GC Search Trop Picture | |
| pepper bell pepper fruit Search Trop Picture | |
| peppermint leaf Search Trop Picture | |
| peppermint oil america @ 1.38% Data GC Search Trop Picture | |
| peppermint oil CO2 extract @ 0.30% Data GC Search Trop Picture | |
| peppermint oil mongolia @ 0.22% Data GC Search Trop Picture | |
| peppermint oil russia @ 0.99% Data GC Search Trop Picture | |
| rosemary Search Trop Picture | |
| rue oil cuba @ 0.10% Data GC Search Trop Picture | |
| sage oil cuba @ 0.86% Data GC Search Trop Picture | |
| salvia officinalis oil cuba @ 0.86% Data GC Search Trop Picture | |
| satureja viminea l. oil costa rica @ 35.30% Data GC Search Trop Picture | |
| spearmint Search Trop Picture | |
| spearmint leaf Search Trop Picture | |
| spearmint oil Search Trop Picture | |
| tea black tea Search Trop Picture | |
| thyme oil wild or creeping pakistan @ 0.21% Data GC Search Trop Picture | |
| water mint leaf Search Trop Picture | |
| water mint shoot Search Trop Picture |
Synonyms:
| cyclohexanone, 5-methyl-2- (1-methylethylidene)-, (R)- | |
| cyclohexanone, 5-methyl-2-(1-methylethylidene)-, (5R)- | |
| cyclohexanone, 5-methyl-2-(1-methylethylidene)-, (R)- | |
| (1R)-(+)-p- | menth-4(8)-en-3-one |
| (1R)-(+)-para- | menth-4(8)-en-3-one |
| (R)-(+)-p- | menth-4(8)-en-3-one |
| (R)-(+)-para- | menth-4(8)-en-3-one |
| (R)-p- | menth-4(8)-en-3-one |
| (R)-para- | menth-4(8)-en-3-one |
| R-(+)-p- | menth-4(8)-en-3-one |
| (1R)-(+)-p- | menth-4,8-en-3-one |
| (1R)-(+)-para- | menth-4,8-en-3-one |
| delta-4,8-p- | menthen-3-one |
| delta-4,8-para- | menthen-3-one |
| (R)-5- | methyl-2-(1-methyl ethylidene) cyclohexanone |
| (R)-5- | methyl-2-(1-methylethylidene)cyclohexanone |
| (5R)-5- | methyl-2-(2-propanyliden)cyclohexanon |
| (5R)-5- | methyl-2-(methylethylidene)cyclohexan-1-one |
| (5R)-5- | methyl-2-(propan-2-ylidene)cyclohexan-1-one |
| (5R)-5- | methyl-2-(propan-2-ylidene)cyclohexanone |
| (R)-5- | methyl-2-(propan-2-ylidene)cyclohexanone |
| (5R)-5- | methyl-2-propan-2-ylidenecyclohexan-1-one |
| (R)-1- | methyl-4-isopropylidene-3-cyclohexanone |
| (R)-3- | methyl-6-isopropylidene cyclohexanone |
| (R)-3- | methyl-6-isopropylidenecyclohexanone |
| (R)-1-iso | propylidene-4-methyl-2-cyclohexanone |
| (R)-2-iso | propylidene-5-methyl-cyclohexanone |
| (5R)-2-iso | propylidene-5-methylcyclohexanone |
| (R)-2-iso | propylidene-5-methylcyclohexanone |
| pulegone | |
| (+)- | pulegone |
| (+)-(R)- | pulegone |
| (R)- | pulegone |
| (R)-(+)- | pulegone |
| D- | pulegone |
| dextro- | pulegone |
| pulegone (ex Pennyroyal) | |
| pulegone dextro natural ex pennyroyal oil | |
| D- | pulegone, natural |










