Articles:
(+/-)-2-(5-methyl-5-vinyl-tetrahydrofuran-2-yl)propionaldehyde
Notes:
Used as a food additive [EAFUS]
| CAS Number: | 67920-63-2 | 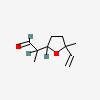 3D/inchi 3D/inchi
|
| FDA UNII: | CZ348GQG4G | |
| Nikkaji Web: | J501.066G | |
| Beilstein Number: | 1618572 | |
| XlogP3-AA: | 1.50 (est) | |
| Molecular Weight: | 168.23592000 | |
| Formula: | C10 H16 O2 | |
| NMR Predictor: | Predict (works with chrome or firefox) | |
| CAS Number: | 51685-39-3 | |
| Molecular Weight: | 168.23592000 | |
| Formula: | C10 H16 O2 | |
Category: flavor and fragrance agents
US / EU / FDA / JECFA / FEMA / FLAVIS / Scholar / Patent Information:
| Google Scholar: | Search |
| Google Books: | Search |
| Google Scholar: with word "volatile" | Search |
| Google Scholar: with word "flavor" | Search |
| Google Scholar: with word "odor" | Search |
| Perfumer and Flavorist: | Search |
| Google Patents: | Search |
| US Patents: | Search |
| EU Patents: | Search |
| Pubchem Patents: | Search |
| PubMed: | Search |
| NCBI: | Search |
| JECFA Food Flavoring: | 1457 (+/-)-2-(5-methyl-5-vinyl-tetrahydrofuran-2-yl)propionaldehyde |
| FEMA Number: | 4058 (+/-)-2-(5-methyl-5-vinyl-tetrahydrofuran-2-yl)propionaldehyde |
| FDA: | No longer provide for the use of these seven synthetic flavoring substances |
| FDA Mainterm (SATF): | 67920-63-2 ; (+/-)-2-(5-METHYL-5-VINYLTETRAHYDROFURAN-2-YL)PROPIONALDEHYDE |
Physical Properties:
| Appearance: | colorless clear liquid (est) |
| Assay: | 90.00 to 100.00 % sum of isomers |
| Food Chemicals Codex Listed: | No |
| Specific Gravity: | 0.95100 to 0.96100 @ 20.00 °C. |
| Pounds per Gallon - (est).: | 7.922 to 8.006 |
| Refractive Index: | 1.45000 to 1.45900 @ 20.00 °C. |
| Boiling Point: | 56.00 to 60.00 °C. @ 1.40 mm Hg |
| Acid Value: | 5.00 max. KOH/g |
| Vapor Pressure: | 0.100000 mmHg @ 25.00 °C. (est) |
| Flash Point: | 173.00 °F. TCC ( 78.40 °C. ) (est) |
| logP (o/w): | 1.591 (est) |
| Soluble in: | |
| alcohol | |
| water, 1229 mg/L @ 25 °C (est) | |
| Insoluble in: | |
| water | |
Organoleptic Properties:
| Odor Type: floral | |
| Odor Strength: | medium |
| floral | |
| Odor Description: at 100.00 %. | floral lilac |
| Odor and/or flavor descriptions from others (if found). | |
Cosmetic Information:
| None found |
Suppliers:
| Parchem |
| (±)-Lilac Aldehyde |
| Chemical Sources Association |
| Need This Item for Flavor/Food?: You can contact the CSA |
Safety Information:
| Hazards identification | |
| Classification of the substance or mixture | |
| GHS Classification in accordance with 29 CFR 1910 (OSHA HCS) | |
| None found. | |
| GHS Label elements, including precautionary statements | |
| Pictogram | |
| Hazard statement(s) | |
| None found. | |
| Precautionary statement(s) | |
| None found. | |
| Oral/Parenteral Toxicity: | |
| Not determined | |
| Dermal Toxicity: | |
| Not determined | |
| Inhalation Toxicity: | |
| Not determined | |
Safety in Use Information:
| Category: | flavor and fragrance agents | ||
| IFRA Code of Practice Notification of the 49th Amendment to the IFRA Code of Practice | |||
| Recommendation for (±)-lilac aldehyde usage levels up to: | |||
| 3.0000 % in the fragrance concentrate. | |||
| Use levels for FEMA GRAS flavoring substances on which the FEMA Expert Panel based its judgments that the substances are generally recognized as safe (GRAS). | |||
| The Expert Panel also publishes separate extensive reviews of scientific information on all FEMA GRAS flavoring substances and can be found at FEMA Flavor Ingredient Library | |||
| publication number: 21 | |||
| Click here to view publication 21 | |||
| average usual ppm | average maximum ppm | ||
| baked goods: | 8.00000 | 16.00000 | |
| beverages(nonalcoholic): | 3.00000 | 6.00000 | |
| beverages(alcoholic): | 4.00000 | 8.00000 | |
| breakfast cereal: | - | - | |
| cheese: | - | - | |
| chewing gum: | - | - | |
| condiments / relishes: | - | - | |
| confectionery froastings: | - | - | |
| egg products: | - | - | |
| fats / oils: | - | - | |
| fish products: | - | - | |
| frozen dairy: | 6.00000 | 12.00000 | |
| fruit ices: | 3.00000 | 6.00000 | |
| gelatins / puddings: | 5.00000 | 10.00000 | |
| granulated sugar: | - | - | |
| gravies: | - | - | |
| hard candy: | 6.00000 | 12.00000 | |
| imitation dairy: | - | - | |
| instant coffee / tea: | - | - | |
| jams / jellies: | - | - | |
| meat products: | - | - | |
| milk products: | - | - | |
| nut products: | - | - | |
| other grains: | - | - | |
| poultry: | - | - | |
| processed fruits: | - | - | |
| processed vegetables: | - | - | |
| reconstituted vegetables: | - | - | |
| seasonings / flavors: | - | - | |
| snack foods: | - | - | |
| soft candy: | 5.00000 | 10.00000 | |
| soups: | - | - | |
| sugar substitutes: | - | - | |
| sweet sauces: | - | - | |
Safety References:
| EPI System: | View |
| Toxicology Citations: | Search |
| EPA ACToR: | Toxicology Data |
| EPA Substance Registry Services (SRS): | Registry |
| Laboratory Chemical Safety Summary : | 155007 |
| National Institute of Allergy and Infectious Diseases: | Data |
| 2-(5-ethenyl-5-methyloxolan-2-yl)propanal | |
| Chemidplus: | 0067920632 |
References:
| Leffingwell: | Chirality or Article |
| 2-(5-ethenyl-5-methyloxolan-2-yl)propanal | |
| NIST Chemistry WebBook: | Search Inchi |
| Pubchem (cid): | 155007 |
| Pubchem (sid): | 135111884 |
| Pubchem (cas): | 51685-39-3 |
Other Information:
| FDA Substances Added to Food (formerly EAFUS): | View |
| CHEBI: | View |
| HMDB (The Human Metabolome Database): | HMDB32434 |
| FooDB: | FDB009879 |
| Typical G.C. | |
| VCF-Online: | VCF Volatile Compounds in Food |
| ChemSpider: | View |
| EFSA Update of results on the monitoring of furan levels in food: | Read Report |
| EFSA Previous report: Results on the monitoring of furan levels in food: | Read Report |
| EFSA Report of the CONTAM Panel on provisional findings on furan in food: | Read Report |
Potential Blenders and core components note
Potential Uses:
| None Found |
Occurrence (nature, food, other): note
| davana oil @ 0.5% Data GC Search Trop Picture | |
| honey Search PMC Picture | |
| lilac Search Trop Picture |
Synonyms:
| 5- | ethenyl tetrahydro-alpha,5-dimethyl-2-furan acetaldehyde |
| 2-(5- | ethenyl-5-methyloxolan-2-yl)propanal |
| 2-(5- | ethenyl-5-methyltetrahydrofuran-2-yl)propanal |
| 5- | ethenyltetrahydro-alpha,5-dimethyl-2-furanacetaldehyde |
| 2- | furanacetaldehyde, 5-ethenyltetrahydro-a,5-dimethyl- |
| (±)-2-(5- | methyl-5-vinyl tetrahydrofuran-2-yl) propionaldehyde |
| (+/-)-2-(5- | methyl-5-vinyl-tetrahydrofuran-2-yl)propionaldehyde |
| 2-(5- | methyl-5-vinyltetrahydro-2-furanyl)propanal |
| 2-(5- | methyl-5-vinyltetrahydrofuran-2-yl)propanal |
| (±)-2-(5- | methyl-5-vinyltetrahydrofuran-2-yl)propionaldehyde |
| 2-(5- | methyl-5-vinyltetrahydrofuran-2-yl)propionaldehyde |
