|
Category: flavor and fragrance agents
US / EU / FDA / JECFA / FEMA / FLAVIS / Scholar / Patent Information:
Physical Properties:
| Appearance: | colorless to pale yellow clear liquid (est) |
| Assay: | 98.10 to 99.30 % sum of isomers
|
| Food Chemicals Codex Listed: | Yes |
| Specific Gravity: | 0.84100 to 0.84800 @ 25.00 °C.
|
| Pounds per Gallon - (est).: | 6.998 to 7.056
|
| Refractive Index: | 1.44500 to 1.44900 @ 20.00 °C.
|
| Boiling Point: | 47.00 °C. @ 17.00 mm Hg
|
| Boiling Point: | 146.00 to 147.00 °C. @ 760.00 mm Hg
|
| Acid Value: | 10.00 max. KOH/g
|
| Vapor Pressure: | 4.624000 mmHg @ 25.00 °C. (est) |
| Vapor Density: | 3.4 ( Air = 1 ) |
| Flash Point: | 101.00 °F. TCC ( 38.33 °C. )
|
| logP (o/w): | 1.790 (est) |
| Shelf Life: | 12.00 month(s) or longer if stored properly. |
| Storage: | refrigerate in tightly sealed containers. |
| Soluble in: |
| | alcohol | | | fixed oils | | | propylene glycol | | | water, 5261 mg/L @ 25 °C (est) |
Organoleptic Properties:
| |
| Odor Type: green |
| |
| Odor Strength: | high ,
recommend smelling in a 1.00 % solution or less |
| |
| Substantivity: | < 8 hour(s) at 10.00 % in dipropylene glycol |
| |
| | green banana aldehydic fatty cheesy |
Odor Description:
at 10.00 % in dipropylene glycol. | green banana aldehydic fatty cheesy
Luebke, William tgsc, (2007) |
| |
| | sharp fresh leafy green clean fruity herbal spicy herbal |
Odor Description:
at 1.00 %. | Sharp, penetrating fresh leafy green, clean, fruity with herbal and spicy herbal nuances
Mosciano, Gerard P&F 25, No. 6, 26, (2000) |
| |
| |
| Flavor Type: green |
| |
| | fresh green leafy fruity vegetable |
Taste Description:
at 0.10 - 5.00 ppm. | Fresh green, leafy, fruity with rich vegetative nuances
Mosciano, Gerard P&F 25, No. 6, 26, (2000) |
| |
| Odor and/or flavor descriptions from others (if found). |
| |
| Bedoukian Research |
| trans-2-HEXENAL (LEAF ALDEHYDE) ≥98.0%, FCC, Kosher |
| Odor Description: | green, citrus, spicy
Imparts fresh, green, and natural topnote in fruity floral types. |
| Taste Description: | Sweet, green apple
Apple, berry, and other fruit flavors. Also citrus flavors, especially orange juice. |
| |
| Moellhausen |
| trans-2-HEXENYL ALDEHYDE |
| Odor Description: | green, leafy, slightly spicy |
| Taste Description: | leafy, fruity, fatty, apple banana, strawberry note |
| |
| Firmenich |
| TRANS-2-HEXENAL NAT Kosher for flavor |
| Taste Description: | Very green, fruity, green apple, leafy with a typical aldehydic
trans-2-HEXENAL brings freshness to a broad range of fruit and vegetable applications. Essential for apple, peach, red fruits, plum and tomato flavors. |
| |
| Pell Wall Perfumes |
| trans-2-Hexenal 0.1% |
| Odor Description: | Leafy-green, fruity-apple, sweet, fresh . Diffusive
According to Arctander it has a: “Powerful green-fruity, pungent vegetabie-like odor, pungent in high concentrations, almost acrylic-sharp, but pleasant fruity and fresh-green in dilutions below 0.1%. Used in perfume compositions as part of fresh-green, natural topnote complexes, often in delicately floral or fruity fragrance types, and in general as a fresh top note” He goes on to suggest that it works exceptionally well with the very sweet materials such as Ethyl Maltol, Strawberry Furanone and so on. |
| |
| FCI SAS |
| TRANS-2-HEXENAL |
| Odor Description: | Powerful green vegetable-like |
| Taste Description: | somewhat fruity with fresh green fruity |
| |
| Hermitage Oils |
| Trans 2 Hexenal Natural Isolate |
| Odor Description: | characteristic
Eleonora Scalseggi has this to say “Also called “leaf aldehyde” this natural isolate has an outstandingly high odour strength, to such an extent that we recommend not to smell it directly from the bottle but to dilute it down to at least 20% because it is extremely pungent at high concentrations. However, once heavily diluted down this material displays a very pleasant green-leafy, oily aroma with fruity-green banana and apple pips nuances. The aroma becomes even fruitier with clean, fresh green apple notes when further diluted down to 1% or less. |
| |
| |
Cosmetic Information:
Suppliers:
| ACS International |
| Hexenal-trans-2 Nat.
|
| Operational Capabilities |
| Advanced Biotech |
| TRANS 2 HEXENAL 10% ETOH NATURAL
Odor: Alcoholic, Strawberry |
| Advanced Biotech |
| TRANS 2 HEXENAL NATURAL
95% min. Odor: Apple, Strawberry |
| Advanced Biotech |
| TRANS 2-HEXENAL 5% ETOH NATURAL
Odor: Alcoholic, Strawberry |
| Advanced Biotech |
| TRANS-2-HEXENAL 50% ETOH NATURAL
Odor: Alcoholic leafy |
| Alfrebro |
| trans-2-HEXENAL NATURAL (1 kg)
Odor: Strong, Fruity, Green, Vegetable |
| Alfrebro |
| trans-2-HEXENAL NATURAL (5 kg)
|
| Alfrebro |
| trans-2-HEXENAL NATURAL 50% in ETHANOL
Odor: Sweet, Fruity, Green Apple, Vegetable |
| Ambles Nature et Chimie |
| TRANS 2 HEXENAL NAT
|
| Apple Flavor & Fragrance |
| trans-2-Hexen-1-al
|
| Aromiens International |
| Trans-2-Hexenal, Natural
|
| Artiste |
| trans-2-Hexenal Natural
|
| Augustus Oils |
| Trans 2 Hexenal
|
| Services |
| Aurochemicals |
| trans-2-HEXENAL, Natural
|
| Axxence Aromatic |
| TRANS-2-HEXENAL 96%, Natural
Kosher |
| Sustainability |
| Bedoukian Research |
| trans-2-HEXENAL (LEAF ALDEHYDE)
≥98.0%, FCC, Kosher Odor: green, citrus, spicy Use: Imparts fresh, green, and natural topnote in fruity floral types. Flavor: Sweet, green apple Apple, berry, and other fruit flavors. Also citrus flavors, especially orange juice. |
| Beijing Lys Chemicals |
| trans-2-Hexenal, Natural
|
| Berjé |
| trans-2-hexenal
|
| Media |
| BOC Sciences |
| For experimental / research use only. |
| trans-2-HEXENAL (LEAF ALDEHYDE) FCC 98.0%
|
| Charkit Chemical |
| HEXENAL, TRANS-2- H0550 FEMA 2560
|
| Citrus and Allied Essences |
| trans-2-Hexenal FCC
|
| Market Report |
| CJ Latta & Associates |
| Natural Trans-2-Hexenal
|
| CJ Latta & Associates |
| TRANS-2-HEXENAL
|
| De Monchy Aromatics |
| trans-2-Hexenal, Natural
|
| EMD Millipore |
| For experimental / research use only. |
| trans-2-Hexenal
|
| Ernesto Ventós |
| TRANS-2-HEXENAL NATURAL FIRMENICH 947867
|
| Ernesto Ventós |
| TRANS-2-HEXENAL NATURAL
|
| Ernesto Ventós |
| TRANS-2-HEXENAL
Odor: GREEN, FRUITY, CITRUS Flavor: GREEN, CITRUS, APPLE |
| Excellentia International |
| trans-2-Hexenal Natural
|
| FCI SAS |
| TRANS-2-HEXENAL
Odor: Powerful green vegetable-like Flavor: somewhat fruity with fresh green fruity |
| Firmenich |
| TRANS-2-HEXENAL NAT Kosher
for flavor Flavor: Very green, fruity, green apple, leafy with a typical aldehydic trans-2-HEXENAL brings freshness to a broad range of fruit and vegetable applications. Essential for apple, peach, red fruits, plum and tomato flavors. |
| Fleurchem |
| trans-2-hexenal natural
|
| Frutarom |
| TRANS 2 HEXENAL
KOSHER Flavor: Aldehydic, Apple, Fruity, Green |
| CBD Offering |
| Glentham Life Sciences |
| trans-2-Hexenal
|
| Hermitage Oils |
| Trans 2 Hexenal Natural Isolate
Odor: characteristic Use: Eleonora Scalseggi has this to say “Also called “leaf aldehyde” this natural isolate has an outstandingly high odour strength, to such an extent that we recommend not to smell it directly from the bottle but to dilute it down to at least 20% because it is extremely pungent at high concentrations. However, once heavily diluted down this material displays a very pleasant green-leafy, oily aroma with fruity-green banana and apple pips nuances. The aroma becomes even fruitier with clean, fresh green apple notes when further diluted down to 1% or less. |
| IFF |
| TRANS 2 HEXENAL
KOSHER Flavor: Aldehydic, Apple, Fruity, Green |
| Indenta Group |
| trans-2-Hexenal
|
| Indukern F&F |
| TRANS-2-HEXENAL NATURAL
Odor: STRONG, FRUITY, GREEN, VEGETABLE |
| Indukern F&F |
| TRANS-2-HEXENAL
Odor: POWERFUL, GREEN, FRUITY, SPICY |
| Inoue Perfumery |
| TRANS-2-HEXENAL
|
| Jiangyin Healthway |
| Trans-2-Hexenal Natural99%
|
| New functional food ingredients |
| Jinan Enlighten Chemical Technology(Wutong Aroma ) |
| trans-2-Hexenal, Kosherk
|
| Keva |
| TRANS-2-HEXENAL
Odor: Grassy, herbaceous, fruity ester like |
| Kingchem Laboratories |
| T2 HEXENAL
Odor: Powerful, green vegetable-like |
| Lluch Essence |
| TRANS-2-HEXENAL ® PFW
|
| Lluch Essence |
| TRANS-2-HEXENAL NATURAL
|
| Lluch Essence |
| TRANS-2-HEXENAL
|
| M&U International |
| NATtrans-2-HEXENAL
|
| M&U International |
| trans-2-Hexenal, Kosher
|
| Moellhausen |
| trans-2-HEXENYL ALDEHYDE
Odor: green, leafy, slightly spicy Flavor: leafy, fruity, fatty, apple banana, strawberry note |
| Natural Advantage |
| Hexenal, trans-2- Nat
Flavor: grassy, green, leafy |
| Riverside Aromatics LTD.is the exclusive distributor for Europe in UK for any non-US based inquiries |
| O'Laughlin Industries |
| TRANS-2-HEXENAL (Leaf aldehyde), US NAT
|
| O'Laughlin Industries |
| TRANS-2-HEXENAL
|
| Oamic Ingredients |
| Trans 2-Hexenal
Odor: Strong, Fruity-grenen, Vegetable |
| OQEMA |
| trans-2-Hexenal natural
|
| OQEMA |
| trans-2-Hexenal natural
|
| PCW France |
| Trans-2-Hexenal
|
| Steps to a fragranced product |
| Pell Wall Perfumes |
| trans-2-Hexenal 0.1%
Odor: Leafy-green, fruity-apple, sweet, fresh . Diffusive Use: According to Arctander it has a: “Powerful green-fruity, pungent vegetabie-like odor, pungent in high concentrations, almost acrylic-sharp, but pleasant fruity and fresh-green in dilutions below 0.1%. Used in perfume compositions as part of fresh-green, natural topnote complexes, often in delicately floral or fruity fragrance types, and in general as a fresh top note” He goes on to suggest that it works exceptionally well with the very sweet materials such as Ethyl Maltol, Strawberry Furanone and so on. |
| Penta International |
| TRANS-2-HEXENAL 50% NATURAL
|
| Penta International |
| TRANS-2-HEXENAL NATURAL
|
| Penta International |
| TRANS-2-HEXENAL
|
| Phoenix Aromas & Essential Oils |
| Trans-2-Hexenal Natural
|
| Prodasynth |
| TRANS-2-HEXENAL
(> 98%) Odor: GREEN, FRUITY, CITRUS Flavor: GREEN, CITRUS, APPLE |
| R C Treatt & Co Ltd |
| trans-2-Hexenal
|
| Reincke & Fichtner |
| trans-2-Hexenal natural
|
| Reincke & Fichtner |
| trans-2-Hexenal
|
| Riverside Aromatics |
| trans -2-HEXENAL, NATURAL
|
| Riverside Aromatics |
| trans-2-HEXENAL
|
| Robertet |
| trans-2-HEXENAL
Pure & Nat (EU) |
| Seasons and Harvest / Crop calendar |
| Santa Cruz Biotechnology |
| For experimental / research use only. |
| trans-2-Hexen-1-al
|
| Sigma-Aldrich |
| trans-2-Hexen-1-al, ≥95%, FCC, FG
Odor: almond; apple; green; plum; sweet; vegetable |
| Certified Food Grade Products |
| Sigma-Aldrich |
| trans-2-Hexen-1-al, natural, ≥95%, FG
|
| SRS Aromatics |
| TRANS-2-HEXENAL EU NATURAL
|
| SRS Aromatics |
| TRANS-2-HEXENAL
|
| Sunaux International |
| nat.trans-2-Hexenal
|
| Sunaux International |
| trans-2-Hexenal
|
| Synerzine |
| TRANS-2-HEXENAL
|
| Taytonn ASCC |
| Trans-2-hexenal
Odor: Citrus, Fruity, Green, Leafy/ Leaf |
| TCI AMERICA |
| For experimental / research use only. |
| trans-2-Hexenal >97.0%(GC)
|
| Tengzhou Jitian Aroma Chemiclal |
| trans-2-Hexenal
|
| The John D. Walsh Company |
| Trans-2-Hexenal
|
| The Lermond Company |
| TRANS 2 HEXENAL
|
| Tianjin Danjun International |
| Natural Trans-2-hexenal
|
| United International |
| Trans-2-Hexenal
|
| Vigon International |
| Hexenal Trans-2 FCC Natural
|
| Vigon International |
| Hexenal Trans-2 FCC
|
| Vigon International |
| HEXENAL TRANS-2 NATURAL 10% IN ETHYL ALCOHOL
|
| Vigon International |
| HEXENAL TRANS-2 NATURAL 5% IN ETHYL ALCOHOL
|
| Vigon International |
| HEXENAL TRANS-2 NATURAL 50% IN ETHYL ALCOHOL
|
| WEN International |
| TRANS-2-HEXENAL Natural
|
Safety Information:
| Preferred SDS: View |
| European information : |
| Most important hazard(s): | | Xn - Harmful. |
R 10 - Flammable.
R 21/22 - Harmful in contact with skin and if swallowed.
R 36/37 - Irritating to eyes and respiratory system.
R 43 - May cause sensitisation by skin contact.
S 02 - Keep out of the reach of children.
S 16 - Keep away from sources of ignition - No Smoking.
S 20/21 - When using do not eat, drink or smoke.
S 24/25 - Avoid contact with skin and eyes.
S 26 - In case of contact with eyes, rinse immediately with plenty of water and seek medical advice.
S 36/37/39 - Wear suitable clothing, gloves and eye/face protection.
|
| |
| Hazards identification |
| |
| Classification of the substance or mixture |
| GHS Classification in accordance with 29 CFR 1910 (OSHA HCS) |
| None found. |
| GHS Label elements, including precautionary statements |
| |
| Pictogram | |
| |
| Hazard statement(s) |
| None found. |
| Precautionary statement(s) |
| None found. |
| Human Experience: |
| 4 % solution: no irritation or sensitization. |
| Oral/Parenteral Toxicity: |
oral-rat LD50 780 mg/kg
VASCULAR: REGIONAL OR GENERAL ARTERIOLAR OR VENOUS DILATION
SENSE ORGANS AND SPECIAL SENSES: LACRIMATION: EYE
GASTROINTESTINAL: CHANGES IN STRUCTURE OR FUNCTION OF SALIVARY GLANDS
Food and Cosmetics Toxicology. Vol. 9, Pg. 775, 1971.
intraperitoneal-rat LD50 180 mg/kg
VASCULAR: REGIONAL OR GENERAL ARTERIOLAR OR VENOUS DILATION
SENSE ORGANS AND SPECIAL SENSES: LACRIMATION: EYE
GASTROINTESTINAL: CHANGES IN STRUCTURE OR FUNCTION OF SALIVARY GLANDS
Food and Cosmetics Toxicology. Vol. 9, Pg. 775, 1971.
oral-mouse LD50 685 mg/kg
LUNGS, THORAX, OR RESPIRATION: DYSPNEA
BEHAVIORAL: SOMNOLENCE (GENERAL DEPRESSED ACTIVITY)
Yukagaku. Oil Chemistry. Vol. 26, Pg. 169, 1977.
intraperitoneal-mouse LD50 100 mg/kg
VASCULAR: REGIONAL OR GENERAL ARTERIOLAR OR VENOUS DILATION
SENSE ORGANS AND SPECIAL SENSES: LACRIMATION: EYE
GASTROINTESTINAL: CHANGES IN STRUCTURE OR FUNCTION OF SALIVARY GLANDS
Food and Cosmetics Toxicology. Vol. 9, Pg. 775, 1971.
|
| Dermal Toxicity: |
skin-rabbit LD50 600 mg/kg
Food and Cosmetics Toxicology. Vol. 13, Pg. 453, 1975.
|
| Inhalation Toxicity: |
|
Not determined
|
Safety in Use Information:
| Category: | flavor and fragrance agents |
| RIFM Fragrance Material Safety Assessment: Search |
| IFRA Code of Practice Notification of the 49th Amendment to the IFRA Code of Practice |
| IFRA Critical Effect: | Dermal sensitization and systemic toxicity |
| IFRA fragrance material specification: | | | Should not be used such that the level in finished cosmetic products
exceeds 0.002%. Based on test results of RIFM showing sensitisation
reactions at 0.2% and no sensitisation reactions when tested at 0.02%
(IFRA guidelines). |
| IFRA: | View Standard |
| View IFRA Standards Library for complete information. |
| Please review Amendment 49 IFRA documentation for complete information. |
| IFRA RESTRICTION LIMITS IN THE FINISHED PRODUCT (%): |
| Category 1: Products applied to the lips |
| 0.0018 % |
| Category 2: Products applied to the axillae |
| 0.00055 % |
| Category 3: Products applied to the face/body using fingertips |
| 0.011 % |
| Category 4: Products related to fine fragrance |
| 0.010 % |
| | Category 5: Products applied to the face and body using the hands (palms), primarily leave-on |
| Category 5A: Body lotion products applied to the body using the hands (palms), primarily leave-on |
| 0.0026 % |
| Category 5B: Face moisturizer products applied to the face using the hands (palms), primarily leave-on |
| 0.0026 % |
| Category 5C: Hand cream products applied to the hands using the hands (palms), primarily leave-on |
| 0.0026 % |
| Category 5D: Baby Creams, baby Oils and baby talc |
| 0.00087 % |
| Category 6: Products with oral and lip exposure |
| 0.0060 % |
| | Category 7: Products applied to the hair with some hand contact |
| Category 7A: Rinse-off products applied to the hair with some hand contact |
| 0.021 % |
| Category 7B: Leave-on products applied to the hair with some hand contact |
| 0.021 % |
| Category 8: Products with significant anogenital exposure |
| 0.00087 % |
| Category 9: Products with body and hand exposure, primarily rinse off |
| 0.020 % |
| | Category 10: Household care products with mostly hand contact |
| Category 10A: Household care excluding aerosol products (excluding aerosol/spray products) |
| 0.020 % |
| Category 10B: Household aerosol/spray products |
| 0.072 % |
| | Category 11: Products with intended skin contact but minimal transfer of fragrance to skin from inert substrate |
| Category 11A: Products with intended skin contact but minimal transfer of fragrance to skin from inert substrate without UV exposure |
| 0.00087 % |
| Category 11B: Products with intended skin contact but minimal transfer of fragrance to skin from inert substrate with potential UV exposure |
| 0.00087 % |
| Category 12: Products not intended for direct skin contact, minimal or insignificant transfer to skin |
| No Restriction |
| | Notes: |
| IFRA FLAVOR REQUIREMENTS: |
Due to the possible ingestion of small amounts of fragrance ingredients from their use in products in Categories 1 and 6, materials must not only comply with IFRA Standards but must also be recognized as safe as a flavoring ingredient as defined by the IOFI Code of Practice (www.iofi.org). For more details see chapter 1 of the Guidance for the use of IFRA Standards. |
| |
| Maximised Survey-derived Daily Intakes (MSDI-EU): | 2800.00 (μg/capita/day) |
| Maximised Survey-derived Daily Intakes (MSDI-USA): | 409.00 (μg/capita/day) |
| Modified Theoretical Added Maximum Daily Intake (mTAMDI): | 1400 (μg/person/day) |
| Threshold of Concern: | 1800 (μg/person/day) |
| Structure Class: | I |
| Use levels for FEMA GRAS flavoring substances on which the FEMA Expert Panel based its judgments that the substances are generally recognized as safe (GRAS). |
| The Expert Panel also publishes separate extensive reviews of scientific information on all FEMA GRAS flavoring substances and can be found at FEMA Flavor Ingredient Library |
| publication number: 3 |
| Click here to view publication 3 |
| | average usual ppm | average maximum ppm |
| baked goods: | - | 16.00000 |
| beverages(nonalcoholic): | - | 3.10000 |
| beverages(alcoholic): | - | - |
| breakfast cereal: | - | - |
| cheese: | - | - |
| chewing gum: | - | - |
| condiments / relishes: | - | - |
| confectionery froastings: | - | - |
| egg products: | - | - |
| fats / oils: | - | - |
| fish products: | - | - |
| frozen dairy: | - | 0.70000 |
| fruit ices: | - | 0.70000 |
| gelatins / puddings: | - | - |
| granulated sugar: | - | - |
| gravies: | - | - |
| hard candy: | - | 15.00000 |
| imitation dairy: | - | - |
| instant coffee / tea: | - | - |
| jams / jellies: | - | - |
| meat products: | - | - |
| milk products: | - | - |
| nut products: | - | - |
| other grains: | - | - |
| poultry: | - | - |
| processed fruits: | - | - |
| processed vegetables: | - | - |
| reconstituted vegetables: | - | - |
| seasonings / flavors: | - | - |
| snack foods: | - | - |
| soft candy: | - | - |
| soups: | - | - |
| sugar substitutes: | - | - |
| sweet sauces: | - | - |
| |
| Food categories according to Commission Regulation EC No. 1565/2000 (EC, 2000) in FGE.06 (EFSA, 2002a). According to the Industry the "normal" use is defined as the average of reported usages and "maximum use" is defined as the 95th percentile of reported usages (EFSA, 2002i). |
| Note: mg/kg = 0.001/1000 = 0.000001 = 1/1000000 = ppm. |
| | average usage mg/kg | maximum usage mg/kg |
| Dairy products, excluding products of category 02.0 (01.0): | 4.64000 | 14.68000 |
| Fats and oils, and fat emulsions (type water-in-oil) (02.0): | - | - |
| Edible ices, including sherbet and sorbet (03.0): | - | - |
| Processed fruit (04.1): | - | - |
| Processed vegetables (incl. mushrooms & fungi, roots & tubers, pulses and legumes), and nuts & seeds (04.2): | - | - |
| Confectionery (05.0): | 4.61000 | 16.41000 |
| Chewing gum (05.0): | - | - |
| Cereals and cereal products, incl. flours & starches from roots & tubers, pulses & legumes, excluding bakery (06.0): | 3.55000 | 8.50000 |
| Bakery wares (07.0): | 5.00000 | 16.87000 |
| Meat and meat products, including poultry and game (08.0): | 1.00000 | 1.00000 |
| Fish and fish products, including molluscs, crustaceans and echinoderms (MCE) (09.0): | - | - |
| Eggs and egg products (10.0): | - | - |
| Sweeteners, including honey (11.0): | - | - |
| Salts, spices, soups, sauces, salads, protein products, etc. (12.0): | - | - |
| Foodstuffs intended for particular nutritional uses (13.0): | - | - |
| Non-alcoholic ("soft") beverages, excl. dairy products (14.1): | 1.95000 | 6.70000 |
| Alcoholic beverages, incl. alcohol-free and low-alcoholic counterparts (14.2): | 1.00000 | 3.00000 |
| Ready-to-eat savouries (15.0): | - | - |
| Composite foods (e.g. casseroles, meat pies, mincemeat) - foods that could not be placed in categories 01.0 - 15.0 (16.0): | - | - |
Safety References:
| Flavor & Extract Manufacturers Association (FEMA) reference(s): |
| The FEMA GRAS assessment of alpha,beta-unsaturated aldehydes and related substances used as flavor ingredients. View pdf |
| European Food Safety Athority(EFSA): | Flavor usage levels; Subacute, Subchronic, Chronic and Carcinogenicity Studies; Developmental / Reproductive Toxicity Studies; Genotoxicity Studies... |
| European Food Safety Authority (EFSA) reference(s): |
List of apha, beta-Unsaturated Aldehydes and Ketones representative of FGE.19 substances for Genotoxicity Testing [1] - Statement of the Panel on Food Contact Materials, Enzymes, Flavourings and Processing Aids (CEF)
View page or View pdf |
EFSA Panel on Food Contact Materials, Enzymes, Flavourings and Processing Aids (CEF)
View page or View pdf |
Scientific Opinion on Flavouring Group Evaluation 200, Revision 1 (FGE.200 Rev.1): 74 a,ß-unsaturated aliphatic aldehydes and precursors from chemical subgroup 1.1.1 of FGE.19
View page or View pdf |
Safety and efficacy of 26 compounds belonging to chemical group 3 (a,ß-unsaturated straight-chain and branched-chain aliphatic primary alcohols, aldehydes, acids and esters) when used as flavourings for all animal species and categories
View page or View pdf |
Scientific Opinion on Flavouring Group Evaluation 71 Revision 1 (FGE.71Rev1): consideration of aliphatic, linear, a,ß-unsaturated alcohols, aldehydes, carboxylic acids, and related esters evaluated by JECFA (63rd and 69th meeting) structurally related to flavouring substances evaluated in FGE.05Rev3
View page or View pdf |
Safety of 31 flavouring compounds belonging to different chemical groups when used as feed additives for all animal species
View page or View pdf |
| EPI System: | View |
| Chemical Carcinogenesis Research Information System: | Search |
| AIDS Citations: | Search |
| Cancer Citations: | Search |
| Toxicology Citations: | Search |
| EPA Substance Registry Services (TSCA): | 6728-26-3 |
| EPA ACToR: | Toxicology Data |
| EPA Substance Registry Services (SRS): | Registry |
| Laboratory Chemical Safety Summary : | 5281168 |
| National Institute of Allergy and Infectious Diseases: | Data |
| SCCNFP: | opinion |
| WISER: | UN 1989 |
| WGK Germany: | 2 |
| | (E)-hex-2-enal |
| Chemidplus: | 0006728263 |
| RTECS: | MP5900000 for cas# 6728-26-3 |
References:
Other Information:
Potential Blenders and core components note
| |
| For Odor |
| No odor group found for these |
| | tea leaf oil | CS |
| acidic |
| iso | butyric acid | FL/FR |
| | cyclohexyl acetic acid | FL/FR |
| | hibiscus rosa-sinensis flower extract | CS |
| 2- | methyl butyric acid | FL/FR |
| alcoholic |
| | fusel oil | FL/FR |
| 3- | hexanol | FL/FR |
| | propyl alcohol | FL/FR |
| aldehydic |
| iso | freshal | FR |
| 3- | methyl-4-(3-methly-3-methoxybutyl oxy) butyraldehyde | FR |
| 3- | methyl-4-octyl oxybutyraldehyde | FR |
| | octane nitrile | FR |
| balsamic |
| | terpinyl isovalerate | FL/FR |
| buttery |
| | acetyl propionyl | FL/FR |
| | butyl butyryl lactate | FL/FR |
| 2,3- | heptane dione | FL/FR |
| cheesy |
| | butyric acid | FL/FR |
| 2- | methyl hexanoic acid | FL/FR |
| S-( | methyl thio) butyrate | FL/FR |
| 2- | methyl valeric acid | FL/FR |
| 2- | methyl-2-hexenoic acid | FR |
| citrus |
| 4- | decenal | FL/FR |
| | methyl heptenone | FL/FR |
| | octanal glycol acetal | FR |
| (E)-2- | tetradecenal | FL/FR |
| | tetrahydrocitral | FL/FR |
| dairy |
| | methyl butyl phenyl acetate | FL/FR |
| earthy |
| | methyl 3-hexenoate | FL/FR |
| ethereal |
| iso | amyl acetoacetate | FL/FR |
| iso | propyl acetate | FL/FR |
| fatty |
| 2,4- | decadien-1-ol | FL/FR |
| | hexanoic acid | FL/FR |
| | methyl (E)-2-hexenoate | FL/FR |
| (S)-gamma- | undecalactone | FL/FR |
| fermented |
| 3- | methyl-1-pentanol | FL/FR |
| 2- | pentanol | FL/FR |
| floral |
| (S)- | citronellyl acetate | FL/FR |
| | citronellyl acetate | FL/FR |
| | citronellyl formate | FL/FR |
| | floral undecenone | FR |
| (E)- | geranyl acetone | FL/FR |
| | geranyl anthranilate | FR |
| | geranyl formate | FL/FR |
| | geranyl propionate | FL/FR |
| | hexyl 2-furoate | FL/FR |
| | neryl isovalerate | FL/FR |
| | phenethyl hexanoate | FL/FR |
| | terpinyl valerate | FL/FR |
| fruity |
| | allyl cyclohexyl propionate | FL/FR |
| | allyl isovalerate | FL/FR |
| iso | amyl 2-methyl butyrate | FL/FR |
| iso | amyl acetate | FL/FR |
| iso | amyl butyrate | FL/FR |
| | amyl hexanoate | FL/FR |
| iso | amyl isobutyrate | FL/FR |
| iso | amyl isovalerate | FL/FR |
| iso | amyl propionate | FL/FR |
| granny smith | apple essence | FL/FR |
| honeycisp | apple essence | FL/FR |
| fuji | apple essence | FL/FR |
| | apple essence | FL/FR |
| ambrosia | apple essence | FL/FR |
| pink lady | apple essence | FL/FR |
| | artemisia pallens herb oil | FL/FR |
| | benzyl propionate | FL/FR |
| iso | butyl (E)-2-butenoate | |
| iso | butyl butyrate | FL/FR |
| | butyl isobutyrate | FL/FR |
| iso | butyl valerate | FL/FR |
| | cyclohexyl acetate | FL/FR |
| | cyclohexyl carboxylic acid | FL/FR |
| | cyclohexyl isovalerate | FL/FR |
| | cyclohexyl propionate | FL/FR |
| | davana oil CO2 extract | FL/FR |
| | diethyl malonate | FL/FR |
| | ethyl butyrate | FL/FR |
| | ethyl hexanoate | FL/FR |
| | ethyl isovalerate | FL/FR |
| | ethyl propionate | FL/FR |
| | filbert hexenone | FL/FR |
| 4- | heptanone | FL/FR |
| alpha- | heptyl cinnamaldehyde | |
| (E)-2- | hexen-1-ol | FL/FR |
| (E)-3- | hexen-1-yl acetate | FL/FR |
| (Z)-3- | hexen-1-yl isobutyrate | FL/FR |
| | hexyl acetate | FL/FR |
| | hexyl isovalerate | FL/FR |
| | methyl 2-methyl butyrate | FL/FR |
| | methyl 2-methyl valerate | FL/FR |
| | methyl 3-nonenoate | FL/FR |
| | methyl 4-methyl valerate | FL/FR |
| 2- | methyl butyl isovalerate | FL/FR |
| | methyl isovalerate | FL/FR |
| | octen-1-yl cyclopentanone | FL/FR |
| | pineapple pentenoate | FL/FR |
| | prenyl hexanoate | FL/FR |
| iso | propyl 2-methyl butyrate | FL/FR |
| | propyl heptanoate | FL/FR |
| | pyrus malus fruit extract | FL/FR |
| | sorbyl butyrate | FL/FR |
| green |
| | acetaldehyde dipropyl acetal | FL/FR |
| | acetaldehyde ethyl phenethyl acetal | FL/FR |
| iso | amyl formate | FL/FR |
| | artichoke leaf | |
| | cilantro leaf oil | FL/FR |
| | cumin acetaldehyde | FL/FR |
| iso | cyclocitral (IFF) | FL/FR |
| | ethyl (E)-2-decenoate | FL/FR |
| | galbanum oil terpeneless | FL/FR |
| | galbanum oleoresin | FL/FR |
| | green carbaldehyde | FR |
| | green note propionate | FL/FR |
| | heptanal (aldehyde C-7) | FL/FR |
| (Z)-3- | hepten-1-yl acetate | FL/FR |
| (Z)-4- | heptenal diethyl acetal | FL/FR |
| | hexanal (aldehyde C-6) | FL/FR |
| (Z)-2- | hexen-1-ol | FL/FR |
| (Z)-3- | hexen-1-yl 2-methyl butyrate | FL/FR |
| (Z)-3- | hexen-1-yl acetate | FL/FR |
| (E)-2- | hexen-1-yl acetate | FL/FR |
| (Z)-3- | hexen-1-yl butyrate | FL/FR |
| (Z)-3- | hexen-1-yl formate | FL/FR |
| (E)-2- | hexen-1-yl hexanoate | FL/FR |
| (E)-2- | hexen-1-yl isovalerate | FL/FR |
| (Z)-3- | hexen-1-yl lactate | FL/FR |
| (Z)-3- | hexen-1-yl propionate | FL/FR |
| (Z)-3- | hexen-1-yl pyruvate | FL/FR |
| (Z)-3- | hexen-1-yl valerate | FL/FR |
| (E)-2- | hexen-1-yl valerate | FL/FR |
| 3- | hexenal | FL/FR |
| 3- | hexenyl 2-methyl butyrate | FL/FR |
| (Z)-3- | hexenyl methyl ether | FR |
| | hexyl 2-methyl butyrate | FL/FR |
| | hexyl heptanoate | FL/FR |
| | hexyl isobutyrate | FL/FR |
| | hexyl octanoate | FL/FR |
| 2,4- | ivy carbaldehyde | FL/FR |
| | leafy oxime | FR |
| | marigold pot absolute | FL/FR |
| | melon nonenoate | FL/FR |
| | methyl (E)-3-hexenoate | FL/FR |
| | neryl butyrate | FL/FR |
| (E,Z)-2,6- | nonadien-1-ol | FL/FR |
| 2,6- | nonadien-1-ol | FL/FR |
| (E,Z)-2,6- | nonadien-1-yl acetate | FL/FR |
| (E)-2- | octen-1-yl acetate | FL/FR |
| 3- | octyl formate | FL/FR |
| | olive oil absolute | FL/FR |
| 1- | penten-3-ol | FL/FR |
| | phenoxyethyl isobutyrate | FL/FR |
| | violet leaf absolute egypt | FL/FR |
| | violet leaf oil (viola odorata) | FL/FR |
| herbal |
| 7- | allyl benzo[b][1,4]dioxepin-3-one | FR |
| iso | amyl heptanoate | FL/FR |
| | linalyl isovalerate | FL/FR |
| minty |
| | peppermint absolute | FL/FR |
| nutty |
| | nutty quinoxaline | FL/FR |
| spicy |
| | cumin seed absolute | FL/FR |
| tea |
| | camellia oleifera leaf extract | FL/FR |
| waxy |
| | ethyl decanoate | FL/FR |
| 2- | methyl heptanoic acid | FL/FR |
| | nonanoic acid | FL/FR |
| 2- | nonanol | FL/FR |
| | octanol | FL/FR |
| | octyl isobutyrate | FL/FR |
| | undecanoic acid | FL/FR |
| |
| For Flavor |
| |
| No flavor group found for these |
| 4- | decenal | FL/FR |
| 2- | decyl furan | FL |
| | ethyl cyclohexyl carboxylate | FL |
| (E,E)-2,4- | heptadien-1-ol | FL |
| (Z)-3- | hepten-1-yl acetate | FL/FR |
| alpha- | heptyl cinnamaldehyde | |
| 2- | hexenal | FL |
| (Z)-3- | hexenoic acid | FL |
| | hexyl heptanoate | FL/FR |
| | methyl (E)-2-hexenoate | FL/FR |
| 5- | methyl hexanoic acid | FL |
| 4- | methyl valeric acid | FL |
| 3- | methyl-3-pentanol | FL |
| | prenyl hexanoate | FL/FR |
| | tetrahydrocitral | FL/FR |
| (S)-gamma- | undecalactone | FL/FR |
| (E)-4- | undecenal | FL |
| acidic |
| iso | butyric acid | FL/FR |
| alcoholic |
| 3- | hexanol | FL/FR |
| | propyl alcohol | FL/FR |
| apple |
| green | apple concentrate | FL |
| red | apple concentrate | FL |
| (E,Z)-2,6- | nonadien-1-ol | FL/FR |
| bitter |
| | terpinyl isovalerate | FL/FR |
| buttery |
| 2,3- | heptane dione | FL/FR |
| 2- | methyl valeric acid | FL/FR |
| (E)-2- | pentenoic acid | FL |
| cheesy |
| | hexanoic acid | FL/FR |
| citrus |
| | cilantro leaf oil | FL/FR |
| cookie |
| apple | cookie flavor | FL |
| creamy |
| | acetoin butyrate | FL |
| | butyl butyryl lactate | FL/FR |
| 2- | methyl-4-pentenoic acid | FL |
| | octyl isobutyrate | FL/FR |
| dairy |
| | methyl butyl phenyl acetate | FL/FR |
| 4- | pentenoic acid | FL |
| ethereal |
| iso | propyl acetate | FL/FR |
| fatty |
| 2,4- | decadien-1-ol | FL/FR |
| | dimethyl sulfoxide | FL |
| | nonanoic acid | FL/FR |
| (E)-2- | octenoic acid | FL |
| 2,4- | undecadienal | FL |
| fermented |
| | methyl thio isovalerate | FL |
| floral |
| | citronellyl acetate | FL/FR |
| (S)- | citronellyl acetate | FL/FR |
| (E)- | geranyl acetone | FL/FR |
| fruity |
| | allyl cyclohexyl propionate | FL/FR |
| | allyl isovalerate | FL/FR |
| iso | amyl 2-methyl butyrate | FL/FR |
| iso | amyl acetate | FL/FR |
| iso | amyl acetoacetate | FL/FR |
| | amyl hexanoate | FL/FR |
| iso | amyl isobutyrate | FL/FR |
| iso | amyl propionate | FL/FR |
| | apple cider flavor | FL |
| | apple concentrate | FL |
| | apple distillates | FL |
| | apple enhancer | FL |
| granny smith | apple essence | FL/FR |
| pink lady | apple essence | FL/FR |
| | apple essence | FL/FR |
| ambrosia | apple essence | FL/FR |
| fuji | apple essence | FL/FR |
| honeycisp | apple essence | FL/FR |
| | apple essence concentrate | FL |
| sour | apple flavor | FL |
| mcintosh | apple flavor | FL |
| red delicious | apple flavor | FL |
| pippin | apple flavor | FL |
| red | apple flavor | FL |
| golden delicious | apple flavor | FL |
| green | apple flavor | FL |
| delicious | apple flavor | FL |
| gala | apple flavor | FL |
| fuji | apple flavor | FL |
| granny smith | apple flavor | FL |
| | apple flavor | FL |
| | apple fractions | FL |
| | apple juice | FL |
| | apple juice concentrate | FL |
| | apple juice flavor | FL |
| | artemisia pallens herb oil | FL/FR |
| | benzyl propionate | FL/FR |
| iso | butyl butyrate | FL/FR |
| | butyl isobutyrate | FL/FR |
| iso | butyl valerate | FL/FR |
| | citronellyl formate | FL/FR |
| | cyclohexyl carboxylic acid | FL/FR |
| | cyclohexyl isovalerate | FL/FR |
| | cyclohexyl propionate | FL/FR |
| | davana oil CO2 extract | FL/FR |
| | diethyl malonate | FL/FR |
| | ethyl (E)-2-decenoate | FL/FR |
| | ethyl butyrate | FL/FR |
| | ethyl hexanoate | FL/FR |
| | ethyl isovalerate | FL/FR |
| | ethyl propionate | FL/FR |
| | filbert hexenone | FL/FR |
| | furfuryl propionate | FL |
| | furfuryl valerate | FL |
| | fusel oil | FL/FR |
| 4- | heptanone | FL/FR |
| (E)-3- | hexen-1-yl acetate | FL/FR |
| (Z)-3- | hexen-1-yl isobutyrate | FL/FR |
| | hexyl acetate | FL/FR |
| | kiwi distillates | FL |
| | linalyl isovalerate | FL/FR |
| | malpighia punicifolia fruit extract | FL |
| | methyl (E)-3-nonenoate | FL |
| | methyl 2-methyl butyrate | FL/FR |
| | methyl 2-methyl valerate | FL/FR |
| | methyl 3-hexenoate | FL/FR |
| | methyl 3-nonenoate | FL/FR |
| | methyl 4-methyl valerate | FL/FR |
| 2- | methyl allyl butyrate | FL |
| 2- | methyl butyl isovalerate | FL/FR |
| 2- | methyl butyric acid | FL/FR |
| | methyl isovalerate | FL/FR |
| | neryl isovalerate | FL/FR |
| | octen-1-yl cyclopentanone | FL/FR |
| | pineapple pentenoate | FL/FR |
| iso | propyl 2-methyl butyrate | FL/FR |
| | pyrus malus fruit extract | FL/FR |
| | pyrus malus fruit juice | FL |
| | salacca zalacca fruit | FL |
| | snake fruit flavor | FL |
| | sorbyl butyrate | FL/FR |
| | terpinyl valerate | FL/FR |
| green |
| | acetaldehyde dipropyl acetal | FL/FR |
| | acetaldehyde ethyl phenethyl acetal | FL/FR |
| iso | amyl formate | FL/FR |
| iso | amyl isovalerate | FL/FR |
| | artichoke leaf | |
| | cumin acetaldehyde | FL/FR |
| iso | cyclocitral (IFF) | FL/FR |
| 2- | ethyl-5-methyl thiophene | FL |
| | galbanum oil terpeneless | FL/FR |
| | galbanum oleoresin | FL/FR |
| | geranyl formate | FL/FR |
| | green note propionate | FL/FR |
| | heptanal (aldehyde C-7) | FL/FR |
| (Z)-4- | heptenal diethyl acetal | FL/FR |
| | hexanal (aldehyde C-6) | FL/FR |
| (Z)-2- | hexen-1-ol | FL/FR |
| (E)-2- | hexen-1-ol | FL/FR |
| (Z)-3- | hexen-1-yl 2-methyl butyrate | FL/FR |
| (Z)-3- | hexen-1-yl acetate | FL/FR |
| (E)-2- | hexen-1-yl acetate | FL/FR |
| (Z)-3- | hexen-1-yl butyrate | FL/FR |
| (Z)-3- | hexen-1-yl formate | FL/FR |
| (E)-2- | hexen-1-yl hexanoate | FL/FR |
| (E)-2- | hexen-1-yl isovalerate | FL/FR |
| (Z)-3- | hexen-1-yl lactate | FL/FR |
| (Z)-3- | hexen-1-yl propionate | FL/FR |
| (Z)-3- | hexen-1-yl pyruvate | FL/FR |
| (E)-2- | hexen-1-yl valerate | FL/FR |
| (Z)-3- | hexen-1-yl valerate | FL/FR |
| 3- | hexenal | FL/FR |
| 3- | hexenyl 2-methyl butyrate | FL/FR |
| | hexyl 2-furoate | FL/FR |
| | hexyl 2-methyl butyrate | FL/FR |
| | hexyl isobutyrate | FL/FR |
| | hexyl isovalerate | FL/FR |
| | hexyl octanoate | FL/FR |
| 2,4- | ivy carbaldehyde | FL/FR |
| | marigold pot absolute | FL/FR |
| | melon nonenoate | FL/FR |
| | methyl (E)-3-hexenoate | FL/FR |
| | methyl heptenone | FL/FR |
| | neryl butyrate | FL/FR |
| 2,6- | nonadien-1-ol | FL/FR |
| (E,Z)-2,6- | nonadien-1-yl acetate | FL/FR |
| (E)-2- | octen-1-yl acetate | FL/FR |
| 1- | penten-3-ol | FL/FR |
| | phenoxyethyl isobutyrate | FL/FR |
| | violet leaf absolute egypt | FL/FR |
| | violet leaf oil (viola odorata) | FL/FR |
| herbal |
| iso | amyl heptanoate | FL/FR |
| meaty |
| 2- | methyl 3-(methyl thio) furan | FL |
| minty |
| | peppermint absolute | FL/FR |
| musty |
| S-( | methyl thio) butyrate | FL/FR |
| 2- | pentanol | FL/FR |
| | propionaldehyde | FL |
| nutty |
| | nutty quinoxaline | FL/FR |
| oily |
| 2- | methyl hexanoic acid | FL/FR |
| | olive oil absolute | FL/FR |
| solvent |
| | cyclohexyl acetate | FL/FR |
| sour |
| | butyric acid | FL/FR |
| | corn apple cider vinegar flavor | FL |
| 2,4- | dimethyl-2-pentenoic acid | FL |
| 3- | methyl valeric acid | FL |
| spicy |
| spiced | apple cider flavor | FL |
| | apple cinnamon french toast flavor | FL |
| | apple spice mandarin flavor | FL |
| | cumin seed absolute | FL/FR |
| sulfurous |
| | methyl 4-(methyl thio) butyrate | FL |
| sweet |
| | cyclohexyl acetic acid | FL/FR |
| tea |
| | camellia oleifera leaf extract | FL/FR |
| toasted |
| | acetyl propionyl | FL/FR |
| waxy |
| iso | amyl butyrate | FL/FR |
| iso | butyl (E)-2-butenoate | |
| | ethyl decanoate | FL/FR |
| | geranyl propionate | FL/FR |
| 2- | methyl heptanoic acid | FL/FR |
| 2- | nonanol | FL/FR |
| | octanol | FL/FR |
| 3- | octyl formate | FL/FR |
| | phenethyl hexanoate | FL/FR |
| | propyl heptanoate | FL/FR |
| (E)-2- | tetradecenal | FL/FR |
| | undecanoic acid | FL/FR |
| whiskey |
| 3- | methyl-1-pentanol | FL/FR |
| |
Potential Uses:
Occurrence (nature, food, other): noteSynonyms:
| (E)- | hex-2-en-1-al | | (2E)- | hex-2-enal | | (E)- | hex-2-enal | | trans- | hex-2-enal | | | hex-2(trans)-enal | | (2E)-2- | hexen-1-al | | (E)-2- | hexen-1-al | | trans-2- | hexen-1-al | | trans-2- | hexen-1-al natural | | (2E)-2- | hexenal | | (E)-2- | hexenal | | 2-trans- | hexenal | | T2 | hexenal | | trans-2- | hexenal | | trans-2- | hexenal (leaf aldehyde) | | trans-2- | hexenal 10% in ETOH natural | | trans-2- | hexenal 5% in ETOH natural | | trans-2- | hexenal 50% in ETOH natural | | trans-2- | hexenal 50% natural | | trans-2- | hexenal FCC | | trans-2- | hexenal nat | | trans-2- | hexenal natural | | | hexenal trans-2 FCC | | | hexenal trans-2 FCC natural | | trans-2- | hexenyl aldehyde | | (E)- | leaf aldehyde | | (E)-beta- | propyl acrolein |
Articles:
| Info: | cis-3-HEXENAL, trans-2-HEXENAL
and 'GREEN GRASS' SMELL |
| PubMed: | HS-SPME-GC-FID method for detection and quantification of Bacillus cereus ATCC 10702 mediated 2-acetyl-1-pyrroline. |
| J-Stage: | Effects of Aliphatic Aldehydes on the Growth and Patulin Production of Penicillium expansum in Apple Juice |
| PubMed: | Bagging treatment influences production of c6 aldehydes and biosynthesis-related gene expression in peach fruit skin. |
| PubMed: | Fractionation and identification of minor and aroma-active constituents in Kangra orthodox black tea. |
| PubMed: | Characterization of the most aroma-active compounds in cherry tomato by application of the aroma extract dilution analysis. |
| PubMed: | A combination of plant-derived odors reduces corticosterone and oxidative indicators of stress. |
| PubMed: | Identification of oxidatively modified proteins in salt-stressed Arabidopsis: a carbonyl-targeted proteomics approach. |
| PubMed: | Evaluating electrophysiological and behavioral responses to volatiles for improvement of odor-baited trap tree management of Conotrachelus nenuphar (Coleoptera: Curculionidae). |
| PubMed: | Aroma active volatiles in four southern highbush blueberry cultivars determined by gas chromatography-olfactometry (GC-O) and gas chromatography-mass spectrometry (GC-MS). |
| PubMed: | Involvement of a specific chemosensory protein from Bactrocera dorsalis in perceiving host plant volatiles. |
| PubMed: | Herbivory-induced plant volatiles from Oryza sativa and their influence on chemotaxis behaviour of Tibraca limbativentris stal. (Hemiptera: Pentatomidae) and egg parasitoids. |
| PubMed: | Chromatographic determination of low-molecular mass unsaturated aliphatic aldehydes with peroxyoxalate chemiluminescence detection after fluorescence labeling with 4-(N,N-dimethylaminosulfonyl)-7-hydrazino-2,1,3-benzoxadiazole. |
| PubMed: | Free and glycosidically bound aroma compounds in cherry (Prunus avium L.). |
| PubMed: | Effect of Addition of Olive Leaves before Fruits Extraction Process to Some Monovarietal Tunisian Extra-Virgin Olive Oils Using Chemometric Analysis. |
| PubMed: | Ultrahigh performance liquid chromatography analysis of volatile carbonyl compounds in virgin olive oils. |
| PubMed: | Chemometric discrimination of different tomato cultivars based on their volatile fingerprint in relation to lycopene and total phenolics content. |
| PubMed: | Silencing in Apolygus lucorum of the olfactory coreceptor Orco gene by RNA interference induces EAG response declining to two putative semiochemicals. |
| PubMed: | In vitro kinetics of soybean lipoxygenase with combinatorial fatty substrates and its functional significance in off flavour development. |
| PubMed: | Atmospheric pressure chemical ionisation mass spectrometry analysis linked with chemometrics for food classification - a case study: geographical provenance and cultivar classification of monovarietal clarified apple juices. |
| PubMed: | Characterization of olfactory receptor neurons for pheromone candidate and plant volatile compounds in the clover root weevil, Sitona lepidus. |
| PubMed: | Flavor of cold-hardy grapes: impact of berry maturity and environmental conditions. |
| PubMed: | The preferential binding of a sensory organ specific odorant binding protein of the alfalfa plant bug Adelphocoris lineolatus AlinOBP10 to biologically active host plant volatiles. |
| PubMed: | Inhibition of bacterial and filamentous fungal growth in high moisture, nonsterile corn with intermittent pumping of trans-2-hexenal vapor. |
| PubMed: | A physiologically based in silico model for trans-2-hexenal detoxification and DNA adduct formation in human including interindividual variation indicates efficient detoxification and a negligible genotoxicity risk. |
| PubMed: | Analyzing blends of herbivore-induced volatile organic compounds with factor analysis: revisiting "cotton plant, Gossypium hirsutum L., defense in response to nitrogen fertilization". |
| PubMed: | Aroma biogenesis and distribution between olive pulps and seeds with identification of aroma trends among cultivars. |
| PubMed: | Volatile composition of six horsetails: prospects and perspectives. |
| PubMed: | Effect of nine plant volatiles in the field on the sex pheromones of Leguminivora glycinivorella. |
| PubMed: | E-2-hexenal promotes susceptibility to Pseudomonas syringae by activating jasmonic acid pathways in Arabidopsis. |
| PubMed: | Odour-active compounds in guava (Psidium guajava L. cv. Red Suprema). |
| PubMed: | New precursor of 3-mercaptohexan-1-ol in grape juice: thiol-forming potential and kinetics during early stages of must fermentation. |
| PubMed: | Effect of storage time and heat processing on the volatile profile of Senegalese sole (Solea senegalensis Kaup, 1858) muscle. |
| PubMed: | Inhibitory effects of fruit flavors on methane production during anaerobic digestion. |
| PubMed: | Effects of exposure to plant-derived odorants on behavior and the concentration of stress-related hormones in steers isolated under a novel environment. |
| PubMed: | Volatile profile and sensory evaluation of tomato juices treated with pulsed electric fields. |
| PubMed: | Effects of aliphatic aldehydes on the growth and patulin production of Penicillium expansum in apple juice. |
| PubMed: | Contribution of ALDH1A1 isozyme to detoxification of aldehydes present in food products. |
| PubMed: | Real-time measurement of volatile chemicals released by bed bugs during mating activities. |
| PubMed: | Activation of the jasmonic acid pathway by depletion of the hydroperoxide lyase OsHPL3 reveals crosstalk between the HPL and AOS branches of the oxylipin pathway in rice. |
| PubMed: | Electrophysiological and behavioral responses of male fall webworm moths (Hyphantria cunea) to Herbivory-induced mulberry (Morus alba) leaf volatiles. |
| PubMed: | Differential performance and parasitism of caterpillars on maize inbred lines with distinctly different herbivore-induced volatile emissions. |
| PubMed: | Influence of deficit irrigation and kaolin particle film on grape composition and volatile compounds in Merlot grape (Vitis vinifera L.). |
| PubMed: | Synergy versus potency in the defensive secretions from nymphs of two pentatomomorphan families (Hemiptera: Coreidae and Pentatomidae). |
| PubMed: | Defensive roles of (E)-2-alkenals and related compounds in heteroptera. |
| PubMed: | Antibacterial activity of 4-oxo-(E)-2-hexenal from adults and nymphs of the heteropteran, Dolycoris baccarum (Heteroptera: Pentatomidae). |
| PubMed: | Plasma membrane potential depolarization and cytosolic calcium flux are early events involved in tomato (Solanum lycopersicon) plant-to-plant communication. |
| PubMed: | An experimental study of the gas-phase reactions of NO3 radicals with a series of unsaturated aldehydes: trans-2-hexenal, trans-2-heptenal, and trans-2-octenal. |
| PubMed: | A physiologically based in silico model for trans-2-hexenal detoxification and DNA adduct formation in rat. |
| PubMed: | Impact of lipid oxidation-derived aldehydes and ascorbic acid on the antioxidant activity of model melanoidins. |
| PubMed: | Enhanced attraction of Plutella xylostella (Lepidoptera: Plutellidae) to pheromone-baited traps with the addition of green leaf volatiles. |
| PubMed: | Functional characterizations of chemosensory proteins of the alfalfa plant bug Adelphocoris lineolatus indicate their involvement in host recognition. |
| PubMed: | Functional characterization of SlscADH1, a fruit-ripening-associated short-chain alcohol dehydrogenase of tomato. |
| PubMed: | Composition of the essential oil of wild growing Artemisia vulgaris from Erie, Pennsylvania. |
| PubMed: | Green leaf volatiles enhance methyl jasmonate response in Arabidopsis. |
| PubMed: | Triacylglycerols composition and volatile compounds of virgin olive oil from Chemlali cultivar: comparison among different planting densities. |
| PubMed: | Volatile generation in bell peppers during frozen storage and thawing using selected ion flow tube mass spectrometry (SIFT-MS). |
| PubMed: | Phytochemicals to suppress Fusarium head blight in wheat-chickpea rotation. |
| PubMed: | Thermotolerant cyclamen with reduced acrolein and methyl vinyl ketone. |
| PubMed: | Naturally occurring norephedrine oxazolidine derivatives in khat (Catha edulis). |
| PubMed: | Aroma chemical composition of red wines from different price categories and its relationship to quality. |
| PubMed: | Functional analysis of general odorant binding protein 2 from the meadow moth, Loxostege sticticalis L. (Lepidoptera: Pyralidae). |
| PubMed: | Electrophysiological and behavioral responses of the black-banded oak borer, Coroebus florentinus, to conspecific and host-plant volatiles. |
| PubMed: | Effects on 3-mercaptohexan-1-ol precursor concentrations from prolonged storage of Sauvignon blanc grapes prior to crushing and pressing. |
| PubMed: | Responses of Dendroctonus brevicomis (Coleoptera: Curculionidae) in behavioral assays: implications to development of a semiochemical-based tool for tree protection. |
| PubMed: | Volatile trans-2-hexenal, a soybean aldehyde, inhibits Aspergillus flavus growth and aflatoxin production in corn. |
| PubMed: | Change of volatile compounds in fresh fish meat during ice storage. |
| PubMed: | Assessment of the validity of maturity metrics for predicting the volatile composition of Concord grape juice. |
| PubMed: | Grape contribution to wine aroma: production of hexyl acetate, octyl acetate, and benzyl acetate during yeast fermentation is dependent upon precursors in the must. |
| PubMed: | Role of pathogen-induced volatiles in the Nicotiana tabacum-Golovinomyces cichoracearum interaction. |
| PubMed: | Electrophysiological and behavioral activity of (E)-2-hexenal in the granary weevil and its application in food packaging. |
| PubMed: | Simultaneous sampling and analysis of indoor air infested with Cimex lectularius L. (Hemiptera: Cimicidae) by solid phase microextraction, thin film microextraction and needle trap device. |
| PubMed: | Volatiles of French ferns and "fougère" scent in perfumery. |
| PubMed: | Effects of carvacrol, (E)-2-hexenal, and citral on the thermal death kinetics of Listeria monocytogenes. |
| PubMed: | Development and characterization of antimicrobial poly(l-lactic acid) containing trans-2-hexenal trapped in cyclodextrins. |
| PubMed: | Olfaction in dragonflies: electrophysiological evidence. |
| PubMed: | Quality evaluation of olive oil by statistical analysis of multicomponent stable isotope dilution assay data of aroma active compounds. |
| PubMed: | Cloning and characterization of AKR4C14, a rice aldo-keto reductase, from Thai Jasmine rice. |
| PubMed: | Evaluation of volatiles from two subtropical strawberry cultivars using GC-olfactometry, GC-MS odor activity values, and sensory analysis. |
| PubMed: | Kinetics studies of the gas-phase reactions of NO3 radicals with series of 1-alkenes, dienes, cycloalkenes, alkenols, and alkenals. |
| PubMed: | Feeding by emerald ash borer larvae induces systemic changes in black ash foliar chemistry. |
| PubMed: | Green odor and depressive-like state in rats: toward an evidence-based alternative medicine? |
| PubMed: | Development of a headspace trap HRGC/MS method for the assessment of the relevance of certain aroma compounds on the sensorial characteristics of commercial apple juice. |
| PubMed: | Effect of enzymes on strawberry volatiles during storage, at different ripeness level, in different cultivars, and during eating. |
| PubMed: | Volatile composition of pomegranates from 9 Spanish cultivars using headspace solid phase microextraction. |
| PubMed: | Effects of bagging on volatiles and polyphenols in "Wanmi" peaches during endocarp hardening and final fruit rapid growth stages. |
| PubMed: | Effect of enzyme activity and frozen storage on jalapeño pepper volatiles by selected ion flow tube-mass spectrometry. |
| PubMed: | Mixture of cis-3-hexenol and trans-2-hexenal attenuates behavioral and stress responses induced by 2,5-dihydro-2,4,5-trimethylthiazoline and electric footshock stress in rats. |
| PubMed: | Fusarium infection in maize: volatile induction of infected and neighboring uninfected plants has the potential to attract a pest cereal leaf beetle, Oulema melanopus. |
| PubMed: | Alarm pheromones and chemical communication in nymphs of the tropical bed bug Cimex hemipterus (Hemiptera: Cimicidae). |
| PubMed: | Effects of transporting and processing Sauvignon blanc grapes on 3-mercaptohexan-1-ol precursor concentrations. |
| PubMed: | Electrophysiological response patterns of 16 olfactory neurons from the trichoid sensilla to odorant from fecal volatiles in the locust, locusta migratoria manilensis. |
| PubMed: | Human aldo-keto reductases 1B1 and 1B10: a comparative study on their enzyme activity toward electrophilic carbonyl compounds. |
| PubMed: | Specific regulation of pyrethrin biosynthesis in Chrysanthemum cinerariaefolium by a blend of volatiles emitted from artificially damaged conspecific plants. |
| PubMed: | Model studies on the pattern of volatiles generated in mixtures of amino acids, lipid-oxidation-derived aldehydes, and glucose. |
| PubMed: | Comparative characterization, expression pattern and function analysis of the 12-oxo-phytodienoic acid reductase gene family in rice. |
| PubMed: | 3-Sulfanylhexanol precursor biogenesis in grapevine cells: the stimulating effect of Botrytis cinerea. |
| PubMed: | Volatile composition and sensory quality of Spanish pomegranates (Punica granatum L.). |
| PubMed: | Cereal crop volatile organic compound induction after mechanical injury, beetle herbivory (Oulema spp.), or fungal infection (Fusarium spp.). |
| PubMed: | Volatile emissions from Alnus glutionosa induced by herbivory are quantitatively related to the extent of damage. |
| PubMed: | Differential response in foliar chemistry of three ash species to emerald ash borer adult feeding. |
| PubMed: | Effectiveness of different solid-phase microextraction fibres for differentiation of selected Madeira island fruits based on their volatile metabolite profile--identification of novel compounds. |
| PubMed: | Different patterns of neuronal activities in the infralimbic and prelimbic cortices and behavioral expression in response to two affective odors, 2,5-dihydro-2,4,5-trimethylthiazoline and a mixture of cis-3-hexenol and trans-2-hexenal, in the freely moving rat. |
| PubMed: | A new method for the determination of carbonyl compounds in wines by headspace solid-phase microextraction coupled to gas chromatography-ion trap mass spectrometry. |
| PubMed: | Environmental stress enhances biosynthesis of flavor precursors, S-3-(hexan-1-ol)-glutathione and S-3-(hexan-1-ol)-L-cysteine, in grapevine through glutathione S-transferase activation. |
| PubMed: | Effects of n-hexanal on dopamine release in the striatum of living rats. |
| PubMed: | Chemical investigation of the essential oil from berries and needles of common juniper (Juniperus communis L.) growing wild in Estonia. |
| PubMed: | Ultrasensitive detection of malondialdehyde with surface-enhanced Raman spectroscopy. |
| PubMed: | Influence of harvest method and period on olive oil composition: an NMR and statistical study. |
| PubMed: | Nymphs of the common bed bug (Cimex lectularius) produce anti-aphrodisiac defence against conspecific males. |
| PubMed: | Effect of the commercial ripening stage and postharvest storage on microbial and aroma changes of 'Ambrunés' sweet cherries. |
| PubMed: | Metals, oxidative stress and neurodegenerative disorders. |
| PubMed: | Relation between developmental stage, sensory properties, and volatile content of organically and conventionally grown pac choi (Brassica rapa var. Mei Qing Choi). |
| PubMed: | Comparison of volatile release in tomatillo and different varieties of tomato during chewing. |
| PubMed: | Impact of harvesting and processing conditions on green leaf volatile development and phenolics in Concord grape juice. |
| PubMed: | Comparison of tomatillo and tomato volatile compounds in the headspace by selected ion flow tube mass spectrometry (SIFT-MS). |
| PubMed: | Expression of genes associated with aroma formation derived from the fatty acid pathway during peach fruit ripening. |
| PubMed: | In vitro growth-inhibitory effect of plant-derived extracts and compounds against Paenibacillus larvae and their acute oral toxicity to adult honey bees. |
| PubMed: | Plant volatiles influence electrophysiological and behavioral responses of Lygus hesperus. |
| PubMed: | 4-oxo-aldehydes from the dorsal abdominal glands of the bed bug (Hemiptera: Cimicidae). |
| PubMed: | Rapid fingerprinting and classification of extra virgin olive oil by microjet sampling and extractive electrospray ionization mass spectrometry. |
| PubMed: | "Green odor" inhalation by stressed rat dams reduces behavioral and neuroendocrine signs of prenatal stress in the offspring. |
| PubMed: | The role of secreting structures position on the leaf volatile organic compounds of Hypericum androsaemum. |
| PubMed: | [Antioxidant properties of essential oils]. |
| PubMed: | Characterization of the antennal olfactory system of the bed bug (Cimex lectularius). |
| PubMed: | The involvement of lipid peroxide-derived aldehydes in aluminum toxicity of tobacco roots. |
| PubMed: | Quantitative comparison of free and bound volatiles of two commercial tomato cultivars (Solanum lycopersicum L.) during ripening. |
| PubMed: | Characteristic aroma-active compounds of Korean perilla (Perilla frutescens Britton) leaf. |
| PubMed: | [Electroantennogram responses of Maruca testulalis (Lepidoptera: Pyralidae) to plant volatiles and sex pheromone]. |
| PubMed: | Activity of natural compounds on Fusarium verticillioides and fumonisin production in stored maize kernels. |
| PubMed: | Role of hydroperoxide lyase in white-backed planthopper (Sogatella furcifera Horváth)-induced resistance to bacterial blight in rice, Oryza sativa L. |
| PubMed: | Between plant and diurnal variation in quantities and ratios of volatile compounds emitted by Vicia faba plants. |
| PubMed: | Plant volatiles regulate the activities of Ca2+ -permeable channels and promote cytoplasmic calcium transients in Arabidopsis leaf cells. |
| PubMed: | [Chemical composition of the virgin oil obtained by mechanical pressing form several grape seed varieties (Vitis vinifera L.) with emphasis on minor constituents]. |
| PubMed: | Evaluation of the toxicity of stress-related aldehydes to photosynthesis in chloroplasts. |
| PubMed: | "Green odor" inhalation by rats down-regulates stress-induced increases in Fos expression in stress-related forebrain regions. |
| PubMed: | Coupling reactions of catechins with natural aldehydes and allyl alcohols and radical scavenging activities of the triglyceride-soluble products. |
| PubMed: | A key volatile infochemical that elicits a strong olfactory response of the predatory mite Neoseiulus californicus, an important natural enemy of the two-spotted spider mite Tetranychus urticae. |
| PubMed: | Addition of alarm pheromone components improves the effectiveness of desiccant dusts against Cimex lectularius. |
| PubMed: | Characterization of volatile substances in apples from Rosaceae family by headspace solid-phase microextraction followed by GC-qMS. |
| PubMed: | Study of the alarming volatile characteristics of Tessaratoma papillosa using SPME-GC-MS. |
| PubMed: | Effect of malaxation conditions on phenol and volatile profiles in olive paste and the corresponding virgin olive oils (Olea europaea L. Cv. Cornicabra). |
| PubMed: | Lipoxygenase gene expression in ripening kiwifruit in relation to ethylene and aroma production. |
| PubMed: | Effect of soybean volatile compounds on Aspergillus flavus growth and aflatoxin production. |
| PubMed: | Encapsulation performance of proteins and traditional materials for spray dried flavors. |
| PubMed: | Response of the egg parasitoids Trissolcus basalis and Telenomus podisi to compounds from defensive secretions of stink bugs. |
| PubMed: | Fragrant unsaturated aldehydes elicit activation of the Keap1/Nrf2 system leading to the upregulation of thioredoxin expression and protection against oxidative stress. |
| PubMed: | Effects of environmental novelty on fear-related behavior and stress responses of rats to emotionally relevant odors. |
| PubMed: | Relating sensory descriptors to volatile components in flavor of specialty rice types. |
| PubMed: | Application of zNosetrade mark for the analysis of selected grape aroma compounds. |
| PubMed: | Analysis of volatiles emitted by potato plants by means of a Colorado beetle electroantennographic detector. |
| PubMed: | A new efficient enantioselective synthesis of (+)-cis-2-methyl-4-propyl-1,3-oxathiane, a valuable ingredient for the aroma of passion fruit. |
| PubMed: | Effects of crop development on the emission of volatiles in leaves of Lycopersicon esculentum and its inhibitory activity to Botrytis cinerea and Fusarium oxysporum. |
| PubMed: | Identification of volatile compounds used in host location by the black bean aphid, Aphis fabae. |
| PubMed: | Direct fungicidal activities of C6-aldehydes are important constituents for defense responses in Arabidopsis against Botrytis cinerea. |
| PubMed: | Adults and nymphs do not smell the same: the different defensive compounds of the giant mesquite bug (Thasus neocalifornicus: Coreidae). |
| PubMed: | Identification of the airborne aggregation pheromone of the common bed bug, Cimex lectularius. |
| PubMed: | Characterization of free flavor compounds in traminette grape and their relationship to vineyard training system and location. |
| PubMed: | Attraction to herbivore-induced plant volatiles by the host-foraging parasitoid fly Exorista japonica. |
| PubMed: | Serotonergic mediation of the antidepressant-like effect of the green leaves odor in mice. |
| PubMed: | Toxicity, DNA binding, and cell proliferation in male F344 rats following short-term gavage exposures to trans-2-hexenal. |
| PubMed: | Effects of direct exposure of green odour components on dopamine release from rat brain striatal slices and PC12 cells. |
| PubMed: | Changes in virgin olive oil quality during low-temperature fruit storage. |
| PubMed: | Toxic oxygenated alpha,beta-unsaturated aldehydes and their study in foods: a review. |
| PubMed: | Aphid and plant volatiles induce oviposition in an aphidophagous hoverfly. |
| PubMed: | Bioactivity and chemical composition of the leaf essential oils of Zanthoxylum rhoifolium and Zanthoxylum setulosum from Monteverde, Costa Rica. |
| PubMed: | Effect of supercritical carbon dioxide decaffeination on volatile components of green teas. |
| PubMed: | The Arabidopsis her1 mutant implicates GABA in E-2-hexenal responsiveness. |
| PubMed: | The effect of various drying techniques on apricot volatiles analysed using direct thermal desorption-GC-TOF/MS. |
| PubMed: | Volatile compounds characterizing Tunisian Chemlali and Chétoui virgin olive oils. |
| PubMed: | Studies on the aroma of maté (Ilex paraguariensis St. Hil.) using headspace solid-phase microextraction. |
| PubMed: | Antimicrobial activity of aroma compounds against Saccharomyces cerevisiae and improvement of microbiological stability of soft drinks as assessed by logistic regression. |
| PubMed: | In vitro and in vivo release of aroma compounds from yellow-fleshed kiwifruit. |
| PubMed: | [Roles of infochemicals in host-selection process of Anastatus japonicus]. |
| PubMed: | Inter- and intraspecific variation in defensive compounds produced by five neotropical stink bug species (Hemiptera: Pentatomidae). |
| PubMed: | "Green odor" inhalation reduces the skin-barrier disruption induced by chronic restraint stress in rats: physiological and histological examinations. |
| PubMed: | Rate coefficients for the reaction of OH with (E)-2-pentenal, (E)-2-hexenal, and (E)-2-heptenal. |
| PubMed: | [Extraction and determination of essential oils in Indocalamus latifolius leaves and Indocalamus tessellatus leaves]. |
| PubMed: | Olfactory receptors on the maxillary palps of small ermine moth larvae: evolutionary history of benzaldehyde sensitivity. |
| PubMed: | Interaction with and effects on the profile of proteins of Botrytis cinerea by C6 aldehydes. |
| PubMed: | High-power ultrasound in olive paste pretreatment. Effect on process yield and virgin olive oil characteristics. |
| PubMed: | An assessment of the role played by some oxidation-related aldehydes in wine aroma. |
| PubMed: | The atmospheric photolysis of E-2-hexenal, Z-3-hexenal and E,E-2,4-hexadienal. |
| PubMed: | Comet fluorescence in situ hybridization analysis for oxidative stress-induced DNA damage in colon cancer relevant genes. |
| PubMed: | Acrolein induces vasodilatation of rodent mesenteric bed via an EDHF-dependent mechanism. |
| PubMed: | Analysis of volatile compounds as spoilage indicators in fresh king salmon (Oncorhynchus tshawytscha) during storage using SPME-GC-MS. |
| PubMed: | Changes in volatile and phenolic compounds with malaxation time and temperature during virgin olive oil production. |
| PubMed: | Discrimination of storage conditions and freshness in virgin olive oil. |
| PubMed: | Phenolic and volatile compounds of extra virgin olive oil (Olea europaea L. Cv. Cornicabra) with regard to fruit ripening and irrigation management. |
| PubMed: | ETR1-, JAR1- and PAD2-dependent signaling pathways are involved in C6-aldehyde-induced defense responses of Arabidopsis. |
| PubMed: | Lipoxygenase involvement in ripening strawberry. |
| PubMed: | In vitro antifungal and anti-elastase activity of some aliphatic aldehydes from Olea europaea L. fruit. |
| PubMed: | On the induction of volatile organic compound emissions by plants as consequence of wounding or fluctuations of light and temperature. |
| PubMed: | Caterpillar-elicited methanol emission: a new signal in plant-herbivore interactions? |
| PubMed: | Determination of 11 low-molecular-weight carbonyl compounds in marine algae by high-performance liquid chromatography. |
| PubMed: | Role of the lipoxygenase/lyase pathway of host-food plants in the host searching behavior of two parasitoid species, Cotesia glomerata and Cotesia plutellae. |
| PubMed: | Critical aspects of the determination of pentafluorobenzyl derivatives of aldehydes by gas chromatography with electron-capture or mass spectrometric detection: Validation of an optimized strategy for the determination of oxygen-related odor-active aldehydes in wine. |
| PubMed: | Activity of trans-2-hexenal against Penicillium expansum in 'Conference' pears. |
| PubMed: | Effects of oxygen and turmeric on the formation of oxidative aldehydes in fresh-pack dill pickles. |
| PubMed: | LC/MS/MS method for the quantitation of trans-2-hexenal-derived exocyclic 1,N(2)-propanodeoxyguanosine in DNA. |
| PubMed: | Diel periodicity in the production of green leaf volatiles by wild and cultivated host plants of stemborer moths, Chilo partellus and Busseola fusca. |
| PubMed: | Orthonasal and retronasal perception of some green leaf volatiles used in beverage flavors. |
| PubMed: | Nonhost angiosperm volatiles and verbenone disrupt response of western pine beetle, Dendroctonus brevicomis (Coleoptera: Scolytidae), to attractant-baited traps. |
| PubMed: | Biosynthesis of trans-2-hexenal in response to wounding in strawberry fruit. |
| PubMed: | Volatiles released from bean plants in response to agromyzid flies. |
| PubMed: | Emission of herbivore-induced volatiles in absence of a herbivore--response of Zea mays to green leaf volatiles and terpenoids. |
| PubMed: | Variations in CYP74B2 (hydroperoxide lyase) gene expression differentially affect hexenal signaling in the Columbia and Landsberg erecta ecotypes of Arabidopsis. |
| PubMed: | Increased EAG responses of tortricid moths after prolonged exposure to plant volatiles: evidence for octopamine-mediated sensitization. |
| PubMed: | Enzymatic hydrogenation of trans-2-nonenal in barley. |
| PubMed: | Host-plant finding by the asparagus fly, Plioreocepta poeciloptera (Diptera: Tephritidae), a monophagous, monovoltine tephritid. |
| PubMed: | Discrimination of olive oils and fruits into cultivars and maturity stages based on phenolic and volatile compounds. |
| PubMed: | The involvement of volatile infochemicals from spider mites and from food-plants in prey location of the generalist predatory mite Neoseiulus californicus. |
| PubMed: | Influence of rearing conditions on the volatile compounds of cooked fillets of Silurus glanis (European catfish). |
| PubMed: | Short and simple syntheses of 4-oxo-(E)-2-hexenal and homologs: pheromone components and defensive compounds of Hemiptera. |
| PubMed: | Major volatile compounds in head-space above olive oil analysed by selected ion flow tube mass spectrometry. |
| PubMed: | How rainfall, relative humidity and temperature influence volatile emissions from apple trees in situ. |
| PubMed: | Effect of soybean lipoxygenase on volatile generation and inhibition of Aspergillus flavus mycelial growth. |
| PubMed: | Further field evaluation of synthetic herbivore-induced plant volatiles as attractants for beneficial insects. |
| PubMed: | Volatile C6-aldehydes and Allo-ocimene activate defense genes and induce resistance against Botrytis cinerea in Arabidopsis thaliana. |
| PubMed: | Carcinogenic potential of trans-2-hexenal is based on epigenetic effect. |
| PubMed: | Rapid determination of C6-aldehydes in tomato plant emission by gas chromatography-mass spectrometry and solid-phase microextraction with on-fiber derivatization. |
| PubMed: | Localization and characterization of glutathione-s-transferase isozymes alpha, mu, and pi within the mouse vomeronasal organ. |
| PubMed: | Changes in aroma volatile compounds and ethylene production during "Hujingmilu" peach (Prunus persica L.) fruit development. |
| PubMed: | Enhancement of attraction to sex pheromones of Spodoptera exigua by volatile compounds produced by host plants. |
| PubMed: | Electroantennogram responses of the three migratory forms of the damson-hop aphid, Phorodon humuli, to aphid pheromones and plant volatiles. |
| PubMed: | EAG and behavioral responses of Helicoverpa armigera males to volatiles from poplar leaves and their combinations with sex pheromone. |
| PubMed: | Comparison of the olfactory sensitivity of two sympatric steppe grasshopper species (Orthoptera: Acrididae) to plant volatile compounds. |
| PubMed: | [Attraction effect of main volatile components from tea shoots and flowers on Sphaerophoria menthastri (Diptera: Syrphidae) and Chrysopa septempunctata (Neuroptera: Chrysopidae)]. |
| PubMed: | Aroma extract dilution analysis of cv. Meeker (Rubus idaeus L.) red raspberries from Oregon and Washington. |
| PubMed: | Characterization of carrot root oil arising from supercritical fluid carbon dioxide extraction. |
| PubMed: | Electrophysiological characterisation of olfactory sensilla in the black bean aphid, Aphis fabae. |
| PubMed: | Flavor perception and aroma release from model dairy desserts. |
| PubMed: | Composition rather than viscosity modifies the aroma compound retention of flavored stirred yogurt. |
| PubMed: | Formation of volatile compounds in model experiments with crude leek (Allium ampeloprasum Var. Lancelot) enzyme extract and linoleic acid or linolenic acid. |
| PubMed: | Angiosperm bark volatiles disrupt response of Douglas-fir beetle, Dendroctonus pseudotsugae, to attractant-baited traps. |
| PubMed: | Defensive chemistry of an aposematic bug, Pachycoris stallii Uhler and volatile compounds of its host plant Croton californicus Muell.-Arg. |
| PubMed: | Cardiac toxic effects of trans-2-hexenal are mediated by induction of cardiomyocyte apoptotic pathways. |
| PubMed: | Stereochemical course of the generation of 3-mercaptohexanal and 3-mercaptohexanol by beta-lyase-catalyzed cleavage of cysteine conjugates. |
| PubMed: | Analysis of a model reaction system containing cysteine and (E)-2-methyl-2-butenal, (E)-2-hexenal, or mesityl oxide. |
| PubMed: | Characterization of a hydroperoxide lyase gene and effect of C6-volatiles on expression of genes of the oxylipin metabolism in Citrus. |
| PubMed: | Semiochemicals from the predatory stink bug Eocanthecona furcellata (Wolff): components of metathoracic gland, dorsal abdominal gland, and sternal gland secretions. |
| PubMed: | Activation of the anterior cingulate gyrus by 'Green Odor': a positron emission tomography study in the monkey. |
| PubMed: | Volatile constituents of uncooked rhubarb (Rheum rhabarbarum L.) stalks. |
| PubMed: | Alpha,beta-unsaturated carbonyl compounds: induction of oxidative DNA damage in mammalian cells. |
| PubMed: | Identification of volatile compounds in soybean at various developmental stages using solid phase microextraction. |
| PubMed: | Volatile compounds released by disturbed and calm adults of the tarnished plant bug, Lygus lineolaris. |
| PubMed: | Modulation of pumpkin glutathione S-transferases by aldehydes and related compounds. |
| PubMed: | Functional specialisation and polyphenism in aphid olfactory sensilla. |
| PubMed: | Plant odour processing in the antennal lobe of male and female grapevine moths, Lobesia botrana (Lepidoptera: Tortricidae). |
| PubMed: | Changes in the order of elution of some organic compounds in high-resolution gas chromatography on polar columns depending on the injection method and water content in the sample. |
| PubMed: | Application of hexanal, E-2-hexenal, and hexyl acetate to improve the safety of fresh-sliced apples. |
| PubMed: | Putative colon cancer risk factors damage global DNA and TP53 in primary human colon cells isolated from surgical samples. |
| PubMed: | Volatile fractions from three cultivars of Olea europaea L. collected in two different seasons. |
| PubMed: | Volatile components and aroma active compounds in aqueous essence and fresh pink guava fruit puree (Psidium guajava L.) by GC-MS and multidimensional GC/GC-O. |
| PubMed: | Volatiles from leaves, fruits, and virgin oil from Olea europaea Cv. Olivastra Seggianese from Italy. |
| PubMed: | Inhibition of succinic semialdehyde dehydrogenase activity by alkenal products of lipid peroxidation. |
| PubMed: | Behavioral and electrophysiological responses of natural enemies to synomones from tea shoots and kairomones from tea aphids, Toxoptera aurantii. |
| PubMed: | Aldehyde-induced xanthine oxidase activity in raw milk. |
| PubMed: | C6-Green leaf volatiles trigger local and systemic VOC emissions in tomato. |
| PubMed: | Odor-active compounds of Iberian hams with different aroma characteristics. |
| PubMed: | Study on the mechanisms of the antibacterial action of some plant alpha,beta-unsaturated aldehydes. |
| PubMed: | Sensory evaluation of character impact components in an apple model mixture. |
| PubMed: | Application of the porapak q column extraction method for tomato flavor volatile analysis. |
| PubMed: | Electroantennogram responses of Nilaparvata lugens (Homoptera: Delphacidae) to plant volatile compounds. |
| PubMed: | Composition of the volatiles from intact and mechanically pierced tea aphid-tea shoot complexes and their attraction to natural enemies of the tea aphid. |
| PubMed: | Chemical defense in the plant bug Lopidea robiniae (Uhler). |
| PubMed: | Learning of herbivore-induced and nonspecific plant volatiles by a parasitoid, Cotesia kariyai. |
| PubMed: | Characterization of the most odor-active compounds of Iberian ham headspace. |
| PubMed: | Response of plum curculio (Coleoptera: Curculionidae) to odor-baited traps near woods. |
| PubMed: | Aromatic profile of aqueous banana essence and banana fruit by gas chromatography-mass spectrometry (GC-MS) and gas chromatography-olfactometry (GC-O). |
| PubMed: | DNA-damaging potential and glutathione depletion of 2-cyclohexene-1-one in mammalian cells, compared to food relevant 2-alkenals. |
| PubMed: | Phytotoxic and fungitoxic activities of the essential oil of kenaf (Hibiscus cannabinus L.) leaves and its composition. |
| PubMed: | Behavioral and electrophysiological responses of Arhopalus tristis to burnt pine and other stimuli. |
| PubMed: | On-line analysis of reactive VOCs from urban lawn mowing. |
| PubMed: | Olfactory responses of Ips duplicatus from inner Mongolia, China to nonhost leaf and bark volatiles. |
| PubMed: | Effect of trans-2-hexenal on the growth of Aspergillus flavus in relation to its concentration, temperature and water activity. |
| PubMed: | Identification and quantification of impact odorants of aged red wines from Rioja. GC-olfactometry, quantitative GC-MS, and odor evaluation of HPLC fractions. |
| PubMed: | In vitro antibacterial activity of some aliphatic aldehydes from Olea europaea L. |
| PubMed: | Behavioral responses of the diamondback moth, Plutella xylostella, to green leaf volatiles of Brassica oleracea subsp. capitata. |
| PubMed: | Characterization of the glutathione binding site of aldose reductase. |
| PubMed: | Selective protection by stably transfected human ALDH3A1 (but not human ALDH1A1) against toxicity of aliphatic aldehydes in V79 cells. |
| PubMed: | Flavor authenticity studies by (2)h/(1)h ratio determination using on-line gas chromatography pyrolysis isotope ratio mass spectrometry. |
| PubMed: | Physicochemical Characteristics of Selected Sweet Cherry Cultivars. |
| PubMed: | Use of humidified air in optimizing APCI-MS response in breath analysis. |
| PubMed: | Inhibition of formation of oxidative volatile components in fermented cucumbers by ascorbic acid and turmeric. |
| PubMed: | Effect of high-pressure treatment on lipoxygenase activity. |
| PubMed: | Volatiles released from oak, a host tree for the bark beetle Scolytus intricatus. |
| PubMed: | Stabilization of a novel enzyme.substrate intermediate in the Y206F mutant of Candida albicans EBP1: evidence for acid catalysis. |
| PubMed: | Transient kinetics and intermediates formed during the electron transfer reaction catalyzed by Candida albicans estrogen binding protein. |
| PubMed: | 5-Hydroxymethylfurfural: assessment of mutagenicity, DNA-damaging potential and reactivity towards cellular glutathione. |
| PubMed: | Structure-activity relationships in the induction of DNA-protein cross-links by hematotoxic ring-opened benzene metabolites and related compounds in HL60 cells. |
| PubMed: | Effects of hexanal, trans-2-hexenal, and storage temperature on shelf life of fresh sliced apples. |
| PubMed: | Production, regeneration and biochemical precursors of the major components of the defensive secretion of Eurycotis floridana (Dictyoptera, polyzosteriinae). |
| PubMed: | A hairy root culture of melon produces aroma compounds. |
| PubMed: | Kinetic and structural characterization of the glutathione-binding site of aldose reductase. |
| PubMed: | Development of oxidized odor and volatile aldehydes in fermented cucumber tissue exposed to oxygen. |
| PubMed: | Aroma chemicals isolated and identified from leaves of Aloe arborescens Mill. Var. Natalensis Berger. |
| PubMed: | Anti-Helicobacter pylori agents from the cashew apple. |
| PubMed: | Analytical evaluation of virgin olive oil of first and second extraction. |
| PubMed: | Ability of carnosine and other skeletal muscle components to quench unsaturated aldehydic lipid oxidation products. |
| PubMed: | Tandem mass spectrometry of model peptides modified with trans-2-hexenal, a product of lipid peroxidation. |
| PubMed: | An olfactory-specific glutathione-S-transferase in the sphinx moth Manduca sexta. |
| PubMed: | C6-volatiles derived from the lipoxygenase pathway induce a subset of defense-related genes. |
| PubMed: | Alpha,beta-unsaturated aldehydes increase glutathione S-transferase mRNA and protein: correlation with activation of the antioxidant response element. |
| PubMed: | Microbial populations of Botrytis cinerea-inoculated strawberry fruit exposed to four volatile compounds. |
| PubMed: | Odorant response of individual sensilla on the Drosophila antenna. |
| PubMed: | Interactions of alpha, beta-unsaturated aldehydes and ketones with human glutathione S-transferase P1-1. |
| PubMed: | Subcellular Localization of Secondary Lipid Metabolites Including Fragrance Volatiles in Carnation Petals. |
| PubMed: | Modification of horse heart cytochrome c with trans-2-hexenal. |
| PubMed: | Inhibition of glutathione S-transferase activity in human melanoma cells by alpha,beta-unsaturated carbonyl derivatives. Effects of acrolein, cinnamaldehyde, citral, crotonaldehyde, curcumin, ethacrynic acid, and trans-2-hexenal. |
| PubMed: | Green leaf volatiles as antiaggregants for the mountain pine beetle,Dendroctonus ponderosae Hopkins (Coleoptera: Scolytidae). |
| PubMed: | (E)-2-hexenal-induced DNA damage and formation of cyclic 1,N2-(1,3-propano)-2'-deoxyguanosine adducts in mammalian cells. |
| PubMed: | Field attraction ofBiprorulus bibax Breddin (Hemiptera: Pentatomidae) to synthetic aggregation pheromone and (E)-2-hexenal, a pentatomid defense chemical. |
| PubMed: | The impact of alteration of polyunsaturated fatty acid levels on C6-aldehyde formation of Arabidopsis thaliana leaves. |
| PubMed: | Indole and (E)-2-hexenal, phytochemical potentiators of polymyxins against Pseudomonas aeruginosa and Escherichia coli. |
| PubMed: | The influence of glutathione and detoxifying enzymes on DNA damage induced by 2-alkenals in primary rat hepatocytes and human lymphoblastoid cells. |
| PubMed: | Olfactory reception of potential pheromones and plant odors by tarnished plant bug,Lygus lineolaris (Hemiptera: Miridae). |
| PubMed: | Plant-natural enemy association in tritrophic system,Cotesia rubecula-Pieris rapae-brassicaceae (Cruciferae). III: Collection and identification of plant and frass volatiles. |
| PubMed: | Olea europaea chemicals repellent toDacus oleae females. |
| PubMed: | Tasting green leaf volatiles by larvae and adults of Colorado potato beetle,Leptinotarsa decemlineata. |
| PubMed: | Male-specific volatiles from nearctic and Australasian true bugs (Heteroptera: Coreidae and Alydidae). |
| PubMed: | Lipoxygenase-derived aldehydes inhibit fungi pathogenic on soybean. |
| PubMed: | Spring migration of damson-hop aphid,Phorodon humuli (Homoptera, Aphididae), and summer host plant-derived semiochemicals released on feeding. |
| PubMed: | Identification and characterization of deoxyguanosine adducts of mutagenic beta-alkyl-substituted acrolein congeners. |
| PubMed: | Olea europaea Volatiles attractive and repellent to the olive fruit fly (Dacus oleae, Gmelin). |
| PubMed: | Volatile Products of the Lipoxygenase Pathway Evolved from Phaseolus vulgaris (L.) Leaves Inoculated with Pseudomonas syringae pv phaseolicola. |
| PubMed: | Orientation ofMicroplitis croceipes (Hymenoptera: Braconidae) to green leaf volatiles: Dose-response curves. |
| PubMed: | Mutagenicity of beta-alkyl substituted acrolein congeners in the Salmonella typhimurium strain TA100 and genotoxicity testing in the SOS chromotest. |
| PubMed: | Chemistryvis-Ă -vis maternalism in lace bugs (Heteroptera: Tingidae): Alarm pheromones and exudate defense inCorythucha andGargaphia species. |
| PubMed: | Isolation and identification of allelochemicals that attract the larval parasitoid,Cotesia marginiventris (Cresson), to the microhabitat of one of its hosts. |
| PubMed: | Volatile seed germination inhibitors from plant residues. |
| PubMed: | Alarm pheromone of pentatomid bug,Erthesina fullo Thunberg (Hemiptera: Pentatomidae). |
| PubMed: | A pheromone-degrading aldehyde oxidase in the antennae of the moth Manduca sexta. |
| PubMed: | Effects of the pyrrolizidine alkaloid senecionine and the alkenals trans-4-OH-hexenal and trans-2-hexenal on intracellular calcium compartmentation in isolated hepatocytes. |
| PubMed: | Specificity-related suppression of responses to binary mixtures in olfactory receptors of the Colorado potato beetle. |
| PubMed: | Integration of olfactory information in the Colorado potato beetle brain. |
| PubMed: | Strawberry foliage headspace vapor components at periods of susceptibility and resistance toTetranychus urticae Koch. |
| PubMed: | Alcohol Dehydrogenase and Pyruvate Decarboxylase Activity in Leaves and Roots of Eastern Cottonwood (Populus deltoides Bartr.) and Soybean (Glycine max L.). |
| PubMed: | The oxidation of alpha-beta unsaturated aldehydic products of lipid peroxidation by rat liver aldehyde dehydrogenases. |
| PubMed: | In vitro effects of trans-4-hydroxy-2-alkenals on mouse liver cytochrome P-450. |
| PubMed: | Metabolism of the pyrrolizidine alkaloid metabolite trans-4-hydroxy-2-hexenal by mouse liver aldehyde dehydrogenases. |
| PubMed: | Multichemical defense of plant bugHotea gambiae (westwood) (Heteroptera: Scutelleridae) : Sesquiterpenoids from abdominal gland in larvae. |
| PubMed: | Volatile compounds from the predatory insectPodisus maculiventris (Hemiptera: Pentatomidae) : Male and female metathoracic scent gland and female dorsal abdominal gland secretions. |
| PubMed: | Identification of host plant attractants for the carrot fly,Psila rosae. |
| PubMed: | Acute and short-term toxicity studies on trans-2-hexenal. |
| PubMed: | Trans-2-hexenal: mating stimulant for polyphemus moths. |
|
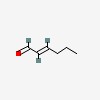 3D/inchi
3D/inchi











































