|
Category: flavor and fragrance agents
US / EU / FDA / JECFA / FEMA / FLAVIS / Scholar / Patent Information:
Physical Properties:
| Appearance: | colorless clear liquid (est) |
| Assay: | 98.00 to 100.00 %
|
| Food Chemicals Codex Listed: | No |
| Specific Gravity: | 0.81900 to 0.82400 @ 25.00 °C.
|
| Pounds per Gallon - (est).: | 6.815 to 6.857
|
| Refractive Index: | 1.41500 to 1.41700 @ 20.00 °C.
|
| Melting Point: | -23.00 °C. @ 760.00 mm Hg
|
| Boiling Point: | 167.00 to 170.00 °C. @ 760.00 mm Hg
|
| Acid Value: | 3.00 max. KOH/g
|
| Vapor Pressure: | 1.504000 mmHg @ 25.00 °C. (est) |
| Flash Point: | 115.00 °F. TCC ( 46.11 °C. )
|
| logP (o/w): | 2.506 (est) |
| Soluble in: |
| | alcohol | | | water, 2600 mg/L @ 20 °C (exp) |
| Insoluble in: |
| | water |
Organoleptic Properties:
| |
| Odor Type: herbal |
| |
| Odor Strength: | medium ,
recommend smelling in a 10.00 % solution or less |
| |
| Substantivity: | > 4 hour(s) at 100.00 % |
| |
| | fresh herbal lavender sweet mushroom |
Odor Description:
at 10.00 % in dipropylene glycol. | fresh herbal lavender sweet mushroom
Luebke, William tgsc, (1988) |
| |
| | musty mushroom ketonic moldy cheesy fermented green vegetable |
Odor Description:
| Musty, mushroom, ketonic, moldy and cheesy fermented with a green, vegetative nuance
Mosciano, Gerard P&F 21, No. 3, 51, (1996) |
| |
| |
| Flavor Type: mushroom |
| |
| | mushroom earthy |
Taste Description:
| mushroom, earthy |
| |
| | mushroom ketonic cheesy moldy fruity |
Taste Description:
at 10.00 ppm. | Mushroom, ketonic, cheesy and moldy with a fruity nuance
Mosciano, Gerard P&F 21, No. 3, 51, (1996) |
| |
| Odor and/or flavor descriptions from others (if found). |
| |
| Bedoukian Research |
| ETHYL AMYL KETONE ≥98.0%, Kosher |
| Odor Description: | Sweet, ripe banana
Used in chypre and carnation compositions. |
| Taste Description: | Characteristic blue cheese, fermented
Very good for a variety of flavor types, including skin notes for peach, fermented notes of mushroom, and also great for pineapple, dairy, and wine. |
| |
| Moellhausen |
| 3-OCTANONE |
| Odor Description: | herbaceous-fruity; slightyly spicy, buttery; topnote of lavender |
| Taste Description: | fatty, green, fruity, waxy, ketonic; mushroom, musty cheese notes |
| |
| PerfumersWorld |
| Ethyl amyl ketone |
| Odor Description: | fresh herbal lavender sweet mushroom herbaceous-fruity slightly spicy buttery lavender
Blends-well-with - Herbaceous-notes Earthy-mossy-notes |
| |
| Firmenich |
| 3-OCTANONE Kosher for flavor |
| Odor Description: | fresh herbal lavender sweet mushroom |
| Taste Description: | Nice fruity, cheesy, mushroom and fatty notes
3-OCTANONE adds a great combination of fruity, cheesy and mushroom notes to vegetables, dairy and exotic fruit flavors |
| |
| |
Cosmetic Information:
Suppliers:
| Alfrebro |
| 3-OCTANONE NATURAL
Odor: Herbaceous, Spicy, Buttery |
| Associate Allied Chemicals |
| Ethyl Amyl Ketone
|
| About |
| Bedoukian Research |
| ETHYL AMYL KETONE
≥98.0%, Kosher Odor: Sweet, ripe banana Use: Used in chypre and carnation compositions. Flavor: Characteristic blue cheese, fermented Very good for a variety of flavor types, including skin notes for peach, fermented notes of mushroom, and also great for pineapple, dairy, and wine. |
| Berjé |
| Ethyl Amyl Ketone
|
| Media |
| CJ Latta & Associates |
| 3-OCTANONE
|
| Diffusions Aromatiques |
| 3-OCTANONE
|
| Ernesto Ventós |
| ETHYL AMYL KETONE
Odor: STRONG, PENETRATING, FRUITY |
| ExtraSynthese |
| For experimental / research use only. |
| 3-Octanone (GC) ≥97%
|
| Firmenich |
| 3-OCTANONE Kosher
for flavor Flavor: Nice fruity, cheesy, mushroom and fatty notes 3-OCTANONE adds a great combination of fruity, cheesy and mushroom notes to vegetables, dairy and exotic fruit flavors |
| Global Essence |
| Ethyl Amyl Ketone
|
| Indukern F&F |
| ETHYL AMYL KETONE
Odor: FRUITY, HERBAL, SPICY |
| Inoue Perfumery |
| AMYL ETHYL KETONE
|
| Lluch Essence |
| ETHYL AMYL KETONE
Odor: FRUITY, SPICY, HERBACEOUS |
| Moellhausen |
| 3-OCTANONE
Odor: herbaceous-fruity; slightyly spicy, buttery; topnote of lavender Flavor: fatty, green, fruity, waxy, ketonic; mushroom, musty cheese notes |
| Nagar Haveli Perfumes & Aromatics |
| Ethyl Amyl Ketone
Natural Odor: Musty, mushroom, ketonic, moldy and cheesy fermented with a green, vegetative nuance |
| Penta International |
| ETHYL AMYL KETONE NATURAL
|
| Penta International |
| ETHYL AMYL KETONE
|
| PerfumersWorld |
| Ethyl amyl ketone
Odor: fresh herbal lavender sweet mushroom herbaceous-fruity slightly spicy buttery lavender Use: Blends-well-with - Herbaceous-notes Earthy-mossy-notes |
| Prodasynth |
| ETHYL AMYL KETONE
(> 98%) Odor: STRONG, PENETRATING, FRUITY |
| R C Treatt & Co Ltd |
| 3-Octanone
|
| Reincke & Fichtner |
| 3-Octanone
|
| Seqens |
| Ethyl N-amyl Ketone, Kosher
|
| Sigma-Aldrich |
| 3-Octanone, ≥98%, FG
Odor: banana; berry; butter; cheese; musty; herbaceous; spicy; green; earthy; vegetable |
| Certified Food Grade Products |
| Som Extracts |
| ETHYL AMYL KETONE
|
| Synerzine |
| 3-Octanone
|
| TCI AMERICA |
| For experimental / research use only. |
| 3-Octanone >98.0%(GC)
|
| Vigon International |
| Ethyl Amyl Ketone (Octanone-3)
Odor: PUNGENT, HERBACEOUS, FRUITY |
Safety Information:
| Preferred SDS: View |
| European information : |
| Most important hazard(s): | | Xi - Irritant |
R 10 - Flammable.
R 36/38 - Irritating to skin and eyes.
S 02 - Keep out of the reach of children.
S 16 - Keep away from sources of ignition - No Smoking.
S 24/25 - Avoid contact with skin and eyes.
S 26 - In case of contact with eyes, rinse immediately with plenty of water and seek medical advice.
S 37/39 - Wear suitable gloves and eye/face protection.
|
| |
| Hazards identification |
| |
| Classification of the substance or mixture |
| GHS Classification in accordance with 29 CFR 1910 (OSHA HCS) |
| None found. |
| GHS Label elements, including precautionary statements |
| |
| Pictogram | |
| |
| Hazard statement(s) |
| None found. |
| Precautionary statement(s) |
| None found. |
| Human Experience: |
| 2 % solution: no irritation or sensitization. |
| Oral/Parenteral Toxicity: |
oral-rat LD50 5000 mg/kg
Shelanski & Moldovan, 1973a
intraperitoneal-mouse LD50 406 mg/kg
Shell Chemical Company. Unpublished Report. Vol. -, Pg. 5, 1961.
|
| Dermal Toxicity: |
skin-rabbit LD50 > 16 mg/kg
Shell Chemical Company. Unpublished Report. Vol. -, Pg. 5, 1961.
|
| Inhalation Toxicity: |
|
Not determined
|
Safety in Use Information:
| Category: | flavor and fragrance agents |
| RIFM Fragrance Material Safety Assessment: Search |
| IFRA Code of Practice Notification of the 49th Amendment to the IFRA Code of Practice |
| Recommendation for 3-octanone usage levels up to: | | | 2.0000 % in the fragrance concentrate.
|
| |
| Maximised Survey-derived Daily Intakes (MSDI-EU): | 2.80 (μg/capita/day) |
| Use levels for FEMA GRAS flavoring substances on which the FEMA Expert Panel based its judgments that the substances are generally recognized as safe (GRAS). |
| The Expert Panel also publishes separate extensive reviews of scientific information on all FEMA GRAS flavoring substances and can be found at FEMA Flavor Ingredient Library |
| publication number: 3 |
| Click here to view publication 3 |
| | average usual ppm | average maximum ppm |
| baked goods: | - | 11.00000 |
| beverages(nonalcoholic): | - | 3.30000 |
| beverages(alcoholic): | - | - |
| breakfast cereal: | - | - |
| cheese: | - | - |
| chewing gum: | - | - |
| condiments / relishes: | - | - |
| confectionery froastings: | - | - |
| egg products: | - | - |
| fats / oils: | - | - |
| fish products: | - | - |
| frozen dairy: | - | 10.00000 |
| fruit ices: | - | 10.00000 |
| gelatins / puddings: | - | - |
| granulated sugar: | - | - |
| gravies: | - | - |
| hard candy: | - | 11.00000 |
| imitation dairy: | - | - |
| instant coffee / tea: | - | - |
| jams / jellies: | - | - |
| meat products: | - | - |
| milk products: | - | - |
| nut products: | - | - |
| other grains: | - | - |
| poultry: | - | - |
| processed fruits: | - | - |
| processed vegetables: | - | - |
| reconstituted vegetables: | - | - |
| seasonings / flavors: | - | - |
| snack foods: | - | - |
| soft candy: | - | - |
| soups: | - | - |
| sugar substitutes: | - | - |
| sweet sauces: | - | - |
Safety References:
| European Food Safety Athority(EFSA): | Flavor usage levels; Subacute, Subchronic, Chronic and Carcinogenicity Studies; Developmental / Reproductive Toxicity Studies; Genotoxicity Studies... |
| European Food Safety Authority (EFSA) reference(s): |
Opinion of the Scientific Panel on food additives, flavourings, processing aids and materials in contact with food (AFC) related to Flavouring Group Evaluation 7 (FGE.07): Saturated and unsaturated aliphatic secondary alcohols, ketones and esters of secondary alcohols and saturated linear or branched-chain carboxylic acids from chemical group 5
View page or View pdf |
Flavouring Group Evaluation 7, Revision 1 (FGE.07Rev1): Saturated and unsaturated aliphatic secondary alcohols, ketones and esters of secondary alcohols and saturated linear or branched-chain carboxylic acids from chemical group 5 (Commission Regulation (EC) No 1565/2000 of 18 July 2000) - Scientific opinion of the Panel on Food Additives, Flavourings, Processing Aids and Materials in contact with Food (AFC) on a request from the Commission
View page or View pdf |
Flavouring Group Evaluation 7, Revision 2 (FGE.07Rev2) : Saturated and unsaturated aliphatic secondary alcohols, ketones and esters of secondary alcohols and saturated linear or branched-chain carboxylic acids from chemical group 5
View page or View pdf |
Safety and efficacy of saturated and unsaturated aliphatic secondary alcohols, ketones and esters with esters containing secondary alcohols belonging to chemical group 5 when used as flavourings for all animal species
View page or View pdf |
| EPI System: | View |
| NIOSH International Chemical Safety Cards: | search |
| NIOSH Pocket Guide: | search |
| Chemical Carcinogenesis Research Information System: | Search |
| AIDS Citations: | Search |
| Cancer Citations: | Search |
| Toxicology Citations: | Search |
| EPA Substance Registry Services (TSCA): | 106-68-3 |
| EPA ACToR: | Toxicology Data |
| EPA Substance Registry Services (SRS): | Registry |
| Laboratory Chemical Safety Summary : | 246728 |
| National Institute of Allergy and Infectious Diseases: | Data |
| WISER: | UN 2271 |
| WGK Germany: | 1 |
| | octan-3-one |
| Chemidplus: | 0000106683 |
| EPA/NOAA CAMEO: | hazardous materials |
| RTECS: | 106-68-3 |
References:
Other Information:
Potential Blenders and core components note
| |
| For Odor |
| No odor group found for these |
| | dehydrolinalool | FL/FR |
| acidic |
| iso | butyric acid | FL/FR |
| | cyclohexyl acetic acid | FL/FR |
| 2- | methyl butyric acid | FL/FR |
| balsamic |
| | balsam ketone | FR |
| (E)- | benzyl tiglate | FL/FR |
| | valerian rhizome absolute | FL/FR |
| buttery |
| | acetyl propionyl | FL/FR |
| | butyl butyryl lactate | FL/FR |
| 2,3- | heptane dione | FL/FR |
| cheesy |
| | butyric acid | FL/FR |
| 2- | methyl hexanoic acid | FL/FR |
| S-( | methyl thio) butyrate | FL/FR |
| 2- | methyl valeric acid | FL/FR |
| 2- | methyl-2-hexenoic acid | FR |
| earthy |
| | dibenzyl ether | FL/FR |
| | heptanal cyclic acetal with glycerol | FL/FR |
| | heptanal glyceryl acetal | FL/FR |
| | methyl undecylenate | FL/FR |
| 1- | nonen-3-ol | FL/FR |
| 3- | octanol | FL/FR |
| 1- | octen-3-ol | FL/FR |
| 1- | octen-3-one | FL/FR |
| fatty |
| (E)-2- | decenal | FL/FR |
| | hexanoic acid | FL/FR |
| | methyl 10-undecenoate | FL/FR |
| fermented |
| | butyl laevo-lactate | FL/FR |
| floral |
| | amyl salicylate | FL/FR |
| 6,8- | dimethyl-2-nonanol | FR |
| | gardenia absolute | FR |
| | herbal pyran | FR |
| | hexyl 2-furoate | FL/FR |
| | lavandin oil | FL/FR |
| | linalyl anthranilate | FL/FR |
| | linalyl propionate | FL/FR |
| | nonisyl propionate | FR |
| | nonyl octanoate | FL/FR |
| | octyl acetate | FL/FR |
| | phenethyl butyl ether | FR |
| 2- | phenyl propionaldehyde dimethyl acetal | FL/FR |
| 2- | phenyl propionaldehyde ethylene glycol acetal | FR |
| | tetrahydrolinalyl acetate | FR |
| fruity |
| iso | amyl butyrate | FL/FR |
| | amyl hexanoate | FL/FR |
| iso | butyl propionate | FL/FR |
| | cyclohexyl carboxylic acid | FL/FR |
| 2,2- | dimethyl-6,8-nonadien-3-ol | |
| endo- | ethyl bicyclo(2.2.1)-5-heptene-2-carboxylate | FR |
| | filbert hexenone | FL/FR |
| | fruity cyclopentanone | FR |
| 4- | heptanone | FL/FR |
| | methyl 4-methyl valerate | FL/FR |
| 2- | methyl butyl isovalerate | FL/FR |
| 2- | nonanone | FL/FR |
| | octyl propionate | FL/FR |
| 4- | phenyl-2-butyl acetate | FL/FR |
| | pineapple pentenoate | FL/FR |
| | prenyl hexanoate | FL/FR |
| | sorbyl butyrate | FL/FR |
| | strawberry furanone butyrate | FL/FR |
| | tropical indene | FR |
| fungal |
| 1- | decen-3-ol | FL/FR |
| | methyl 2-furoate | FL/FR |
| green |
| | butyl lactate | FL/FR |
| | earthy acetal | FL/FR |
| | evernia prunastri lichen | |
| | green carboxylate | FR |
| | green heptenal | FR |
| 3- | heptanone | FL/FR |
| 3- | hepten-2-one | FL/FR |
| (Z)-3- | hexen-1-yl tiglate | FL/FR |
| (E)-2- | hexenal | FL/FR |
| | ivy carbaldehyde / methyl anthranilate schiff's base | FR |
| (Z)- | leaf acetal | FL/FR |
| | melon acetal | FL/FR |
| | phenyl acetaldehyde | FL/FR |
| | phenyl acetaldehyde dimethyl acetal | FL/FR |
| 1- | phenyl-2-pentanol | FL/FR |
| 2- | propenyl-para-cymene | FR |
| 2-iso | propoxy-3(5)-methyl pyrazine | |
| herbal |
| | amber dioxepine | FR |
| | bergamot mint herb oil | FR |
| iso | dihydrolavandulal | FL/FR |
| delta- | elemene | FL/FR |
| | hexanol | FL/FR |
| | lavandin fragrance | FR |
| abrialis | lavandin oil | FL/FR |
| | lavandin oil replacer | FR |
| | lavandin specialty | FR |
| iso | lavandyl acetate | FR |
| | lavender oil terpenes | |
| | marigold oil mexico | FL/FR |
| | methyl hexyl ether | FL/FR |
| | ocimene oxirane | FR |
| honey |
| | phenethyl furoate | FL/FR |
| moldy |
| | strawberry furanone methyl ether | FL/FR |
| 3- | octen-2-ol | FL/FR |
| (R)-1- | octen-3-ol | FL/FR |
| 1- | octen-3-yl butyrate | FL/FR |
| nutty |
| 2,4- | diethyl-5-propyl oxazole | |
| | nutty quinoxaline | FL/FR |
| orris |
| iso | eugenyl formate | FL/FR |
| spicy |
| | carrot weed oil | FL/FR |
| vegetable |
| | methional | FL/FR |
| waxy |
| 2- | methyl heptanoic acid | FL/FR |
| | methyl laurate | FL/FR |
| | methyl octanoate | FL/FR |
| | nonanoic acid | FL/FR |
| 2- | nonanol | FL/FR |
| (Z)-3- | nonen-1-ol | FL/FR |
| 2- | tridecanone | FL/FR |
| | undecanoic acid | FL/FR |
| woody |
| alpha- | farnesene | FL/FR |
| alpha- | farnesene isomer | FL/FR |
| iso | longifolene epoxide | FR |
| yeasty |
| 2- | octen-4-one | FL/FR |
| |
| For Flavor |
| |
| No flavor group found for these |
| iso | butyl amine | FL |
| | dehydrolinalool | FL/FR |
| 2,4- | diethyl-5-propyl oxazole | |
| 2,2- | dimethyl-6,8-nonadien-3-ol | |
| | earthy acetal | FL/FR |
| delta- | elemene | FL/FR |
| | ethyl cyclohexyl carboxylate | FL |
| iso | eugenyl formate | FL/FR |
| | evernia prunastri lichen | |
| (E,E)-2,4- | heptadien-1-ol | FL |
| | heptanal cyclic acetal with glycerol | FL/FR |
| | heptanal glyceryl acetal | FL/FR |
| (Z)-3- | hexenoic acid | FL |
| | lavender oil terpenes | |
| 5- | methyl hexanoic acid | FL |
| tris( | methyl thio) methane | FL |
| 4- | methyl valeric acid | FL |
| | nonyl octanoate | FL/FR |
| (E)-2- | penten-1-ol | FL |
| 2- | pentyl-1-buten-3-one | FL |
| | phenethyl furoate | FL/FR |
| 4- | phenyl-2-butyl acetate | FL/FR |
| | prenyl hexanoate | FL/FR |
| 2-iso | propoxy-3(5)-methyl pyrazine | |
| acidic |
| iso | butyric acid | FL/FR |
| aromatic |
| | amyl salicylate | FL/FR |
| balsamic |
| (E)- | benzyl tiglate | FL/FR |
| buttery |
| | butyl laevo-lactate | FL/FR |
| 2,3- | heptane dione | FL/FR |
| 2- | methyl valeric acid | FL/FR |
| (E)-2- | pentenoic acid | FL |
| caramellic |
| | methyl 2-furoate | FL/FR |
| cheesy |
| | ammonium isovalerate 30% in pg | FL |
| | hexanoic acid | FL/FR |
| 2- | nonanone | FL/FR |
| creamy |
| | acetoin butyrate | FL |
| | butyl butyryl lactate | FL/FR |
| 3- | hepten-2-one | FL/FR |
| 2- | methyl-4-pentenoic acid | FL |
| dairy |
| 4- | pentenoic acid | FL |
| earthy |
| 1- | decen-3-ol | FL/FR |
| 1- | nonen-3-ol | FL/FR |
| 1,8- | octane dithiol | FL |
| 1- | octen-3-one | FL/FR |
| estery |
| | octyl propionate | FL/FR |
| ethereal |
| | methyl hexyl ether | FL/FR |
| fatty |
| | nonanoic acid | FL/FR |
| (E)-2- | octenoic acid | FL |
| 2- | tridecanone | FL/FR |
| fermented |
| | methyl thio isovalerate | FL |
| floral |
| | linalyl anthranilate | FL/FR |
| fruity |
| | amyl hexanoate | FL/FR |
| iso | butyl propionate | FL/FR |
| | cyclohexyl carboxylic acid | FL/FR |
| | dibenzyl ether | FL/FR |
| | filbert hexenone | FL/FR |
| 4- | heptanone | FL/FR |
| | methyl 4-methyl valerate | FL/FR |
| 2- | methyl butyl isovalerate | FL/FR |
| 2- | methyl butyric acid | FL/FR |
| 2- | phenyl propionaldehyde dimethyl acetal | FL/FR |
| 1- | phenyl-2-pentanol | FL/FR |
| | pineapple pentenoate | FL/FR |
| | sorbyl butyrate | FL/FR |
| | strawberry furanone butyrate | FL/FR |
| | valerian rhizome absolute | FL/FR |
| green |
| | butyl lactate | FL/FR |
| | carrot weed oil | FL/FR |
| alpha- | farnesene | FL/FR |
| alpha- | farnesene isomer | FL/FR |
| | hexanol | FL/FR |
| (Z)-3- | hexen-1-yl tiglate | FL/FR |
| (E)-2- | hexenal | FL/FR |
| (E)-3- | hexenoic acid | FL |
| | hexyl 2-furoate | FL/FR |
| (Z)- | leaf acetal | FL/FR |
| | melon acetal | FL/FR |
| | methyl octanoate | FL/FR |
| | phenyl acetaldehyde dimethyl acetal | FL/FR |
| iso | phorone | FL |
| herbal |
| iso | dihydrolavandulal | FL/FR |
| abrialis | lavandin oil | FL/FR |
| | lavandin oil | FL/FR |
| | linalyl propionate | FL/FR |
| | marigold oil mexico | FL/FR |
| honey |
| | phenyl acetaldehyde | FL/FR |
| ketonic |
| 3- | heptanone | FL/FR |
| meaty |
| 2- | methyl 3-(methyl thio) furan | FL |
| moldy |
| | strawberry furanone methyl ether | FL/FR |
| mushroom |
| 3- | octen-2-ol | FL/FR |
| 1- | octen-3-ol | FL/FR |
| (R)-1- | octen-3-ol | FL/FR |
| 1- | octen-3-yl butyrate | FL/FR |
| musty |
| S-( | methyl thio) butyrate | FL/FR |
| 3- | octanol | FL/FR |
| nutty |
| | nutty quinoxaline | FL/FR |
| oily |
| 2- | methyl hexanoic acid | FL/FR |
| sour |
| | butyric acid | FL/FR |
| 2,4- | dimethyl-2-pentenoic acid | FL |
| 3- | methyl valeric acid | FL |
| sulfurous |
| | methyl 4-(methyl thio) butyrate | FL |
| 1-( | methyl thio)-2-butanone | FL |
| 2- | naphthyl mercaptan | FL |
| sweet |
| | cyclohexyl acetic acid | FL/FR |
| toasted |
| | acetyl propionyl | FL/FR |
| tomato |
| | methional | FL/FR |
| vegetable |
| 2- | octen-4-one | FL/FR |
| waxy |
| iso | amyl butyrate | FL/FR |
| (E)-2- | decenal | FL/FR |
| | methyl 10-undecenoate | FL/FR |
| 2- | methyl heptanoic acid | FL/FR |
| | methyl laurate | FL/FR |
| | methyl undecylenate | FL/FR |
| 2- | nonanol | FL/FR |
| (Z)-3- | nonen-1-ol | FL/FR |
| | octyl 2-furoate | FL |
| | octyl acetate | FL/FR |
| | undecanoic acid | FL/FR |
| |
Potential Uses:
Occurrence (nature, food, other): noteSynonyms:
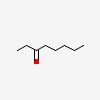
| | amyl ethyl ketone | | N- | amyl ethyl ketone | | | ethyl amyl ketone | | | ethyl N-amyl ketone | | | ethyl N-pentyl ketone | | | ethyl pentyl ketone | | | ethylamylketone | | | heptanone, methyl- | | | ketone, ethyl pentyl | | | oct-3-one | | | octan-3-one | | 3-oxo | octane | | 3- | octanone | | N- | octanone-3 |
Articles:
| US Patents: | 3,952,024 - Furfurylthioacetone |
| PubMed: | Ruthenium Complexes with Dendritic Ferrocenyl Phosphanes: Synthesis, Characterization, and Application in the Catalytic Redox Isomerization of Allylic Alcohols. |
| US Patents: | Certain 2,5-dimethyl-3-thiopyrazines |
| PubMed: | Gas Chromatography-Mass Spectrometry Method Optimized Using Response Surface Modeling for the Quantitation of Fungal Off-Flavors in Grapes and Wine. |
| PubMed: | Attraction of Callosobruchus maculatus (F.) (Coleoptera: Bruchidae) to four varieties of Lathyrus sativus L. seed volatiles. |
| PubMed: | The dynamics of the HS/SPME-GC/MS as a tool to assess the spoilage of minced beef stored under different packaging and temperature conditions. |
| PubMed: | Chemical composition and aroma evaluation of volatile oils from edible mushrooms (Pleurotus salmoneostramineus and Pleurotus sajor-caju). |
| PubMed: | Carbonyl reduction in the biosynthesis of a male sex pheromone secreted by the grape borer Xylotrechus pyrrhoderus. |
| PubMed: | HS/GC-MS analyzed chemical composition of the aroma of fruiting bodies of two species of genus Lentinus (Higher Basidiomycetes). |
| PubMed: | Studies on volatile organic compounds of some truffles and false truffles. |
| PubMed: | Transport of hop aroma compounds across Caco-2 monolayers. |
| PubMed: | The volatome of Aspergillus fumigatus. |
| PubMed: | Identification of volatile biomarkers of gastric cancer cells and ultrasensitive electrochemical detection based on sensing interface of Au-Ag alloy coated MWCNTs. |
| PubMed: | Identification of volatile markers in potato brown rot and ring rot by combined GC-MS and PTR-MS techniques: study on in vitro and in vivo samples. |
| PubMed: | Assessing the chemotaxis behavior of Physarum polycephalum to a range of simple volatile organic chemicals. |
| PubMed: | Evaluation of volatile metabolites as markers in Lycopersicon esculentum L. cultivars discrimination by multivariate analysis of headspace solid phase microextraction and mass spectrometry data. |
| PubMed: | Release and uptake of volatile organic compounds by human hepatocellular carcinoma cells (HepG2) in vitro. |
| PubMed: | Potential aromatic compounds as markers to differentiate between Tuber melanosporum and Tuber indicum truffles. |
| PubMed: | Furry pet allergens, fungal DNA and microbial volatile organic compounds (MVOCs) in the commercial aircraft cabin environment. |
| PubMed: | Can volatile organic metabolites be used to simultaneously assess microbial and mite contamination level in cereal grains and coffee beans? |
| PubMed: | Multiple headspace-solid-phase microextraction: an application to quantification of mushroom volatiles. |
| PubMed: | The Paleobiosphere: a novel device for the in vivo testing of hydrocarbon producing-utilizing microorganisms. |
| PubMed: | Formation yields of C8 1,4-hydroxycarbonyls from OH + n-octane in the presence of NO. |
| PubMed: | [Analyze on volatile compounds of Antrodia camphorata using HS-SPME-GC-MS]. |
| PubMed: | Influence of various growth parameters on fungal growth and volatile metabolite production by indoor molds. |
| PubMed: | Olfactory cues from plants infected by powdery mildew guide foraging by a mycophagous ladybird beetle. |
| PubMed: | Detection of characteristic metabolites of Aspergillus fumigatus and Candida species using ion mobility spectrometry-metabolic profiling by volatile organic compounds. |
| PubMed: | 8-Carbon oxylipins inhibit germination and growth, and stimulate aerial conidiation in Aspergillus nidulans. |
| PubMed: | Different molecular types of Pseudomonas fragi have the same overall behaviour as meat spoilers. |
| PubMed: | Golgi-modifying properties of macfarlandin E and the synthesis and evaluation of its 2,7-dioxabicyclo[3.2.1]octan-3-one core. |
| PubMed: | Volatile organic components from fresh non-edible Basidiomycetes fungi. |
| PubMed: | Olfactory response of Haematobia irritans (Diptera: Muscidae) to cattle-derived volatile compounds. |
| PubMed: | Synthesis of the deuterated sex pheromone components of the grape borer, Xylotrechus pyrrhoderus. |
| PubMed: | Influence of sporophore development, damage, storage, and tissue specificity on the enzymic formation of volatiles in mushrooms (Agaricus bisporus). |
| PubMed: | Antimicrobial activities of components of the glandular secretions of leaf cutting ants of the genus Atta. |
| PubMed: | Use of headspace SPME-GC-MS for the analysis of the volatiles produced by indoor molds grown on different substrates. |
| PubMed: | A GC-MS study of the volatile organic composition of straw and oyster mushrooms during maturity and its relation to antioxidant activity. |
| PubMed: | Induction of conidiation by endogenous volatile compounds in Trichoderma spp. |
| PubMed: | The cytotoxic properties and preferential toxicity to tumour cells displayed by some 2,4-bis(benzylidene)-8-methyl-8-azabicyclo[3.2.1] octan-3-ones and 3,5-bis(benzylidene)-1-methyl-4-piperidones. |
| PubMed: | Correlation between the pattern volatiles and the overall aroma of wild edible mushrooms. |
| PubMed: | Host recognition by the specialist hoverfly Microdon mutabilis, a social parasite of the ant Formica lemani. |
| PubMed: | Genetic diversity of volatile components in Xinjiang Wild Apple (Malus sieversii). |
| PubMed: | Indoor molds, bacteria, microbial volatile organic compounds and plasticizers in schools--associations with asthma and respiratory symptoms in pupils. |
| PubMed: | Differentiation of aroma characteristics of pine-mushrooms (Tricholoma matsutake Sing.) of different grades using gas chromatography-olfactometry and sensory analysis. |
| PubMed: | Asymmetric induction in hydrogen-mediated reductive aldol additions to alpha-amino aldehydes catalyzed by rhodium: selective formation of syn-stereotriads directed by intramolecular hydrogen-bonding. |
| PubMed: | Characterization of aroma-active compounds in raw and cooked pine-mushrooms (Tricholoma matsutake Sing.). |
| PubMed: | Production of volatile compounds by Rhizopus oligosporus during soybean and barley tempeh fermentation. |
| PubMed: | Difference in the volatile composition of pine-mushrooms (Tricholoma matsutake Sing.) according to their grades. |
| PubMed: | Oviposition in Delia platura (Diptera, Anthomyiidae): the role of volatile and contact cues of bean. |
| PubMed: | [GC-MS analysis of volatile oil from the ear of Schizonepeta tenifolia Briq]. |
| PubMed: | Identification of components of male-produced pheromone of coffee white stemborer, Xylotrechus quadripes. |
| PubMed: | Behavioral monitoring of trained insects for chemical detection. |
| PubMed: | Volatile organic compounds in natural biofilm in polyethylene pipes supplied with lake water and treated water from the distribution network. |
| PubMed: | 1,4-hydroxycarbonyl products of the OH radical initiated reactions of C5-C8 n-alkanes in the presence of NO. |
| PubMed: | Main compounds responsible for off-odour of strawberries infected by Phytophthora cactorum. |
| PubMed: | Volatile compounds of Aspergillus strains with different abilities to produce ochratoxin A. |
| PubMed: | Air-water transfer of MTBE, its degradation products, and alternative fuel oxygenates: the role of temperature. |
| PubMed: | Volatile organic compound (VOC) analysis and sources of limonene, cyclohexanone and straight chain aldehydes in axenic cultures of Calothrix and Plectonema. |
| PubMed: | Germination of penicillium paneum Conidia is regulated by 1-octen-3-ol, a volatile self-inhibitor. |
| PubMed: | Volatiles from whitefly-infested plants elicit a host-locating response in the parasitoid, Encarsia formosa. |
| PubMed: | Identification of volatile compounds in soybean at various developmental stages using solid phase microextraction. |
| PubMed: | Field evaluation of potential of alarm pheromone compounds to enhance baits for control of grass-cutting ants (Hymenoptera: Formicidae). |
| PubMed: | Impact odorants contributing to the fungus type aroma from grape berries contaminated by powdery mildew (Uncinula necator); incidence of enzymatic activities of the yeast Saccharomyces cerevisiae. |
| PubMed: | Determination of unique microbial volatile organic compounds produced by five Aspergillus species commonly found in problem buildings. |
| PubMed: | In vivo volatile emissions from peanut plants induced by simultaneous fungal infection and insect damage. |
| PubMed: | Detection and quantification of ochratoxin A and deoxynivalenol in barley grains by GC-MS and electronic nose. |
| PubMed: | A common inhibitory binding site for zinc and odorants at the voltage-gated K(+) channel of rat olfactory receptor neurons. |
| PubMed: | A new passive sampler for regulated workplace ketones. |
| PubMed: | Regulated workplace ketones and their interference in the PFBHA method for aldehydes. |
| PubMed: | Volatiles for mycological quality grading of barley grains: determinations using gas chromatography-mass spectrometry and electronic nose. |
| PubMed: | [MVOC of fungi--use as an indicator for exposure level]. |
| PubMed: | Sensory irritating potency of some microbial volatile organic compounds (MVOCs) and a mixture of five MVOCs. |
| PubMed: | Fungal volatiles as indicators of food and feeds spoilage. |
| PubMed: | Odor detection in rats with 3-methylindole-induced reduction of sensory input. |
| PubMed: | Purification and characterization of two enone reductases from Saccharomyces cerevisiae. |
| PubMed: | Spatially organized response zones in rat olfactory epithelium. |
| PubMed: | Volatile organic chemicals of a shore-dwelling cyanobacterial mat community. |
| PubMed: | Fungal volatiles: Semiochemicals for stored-product beetles (Coleoptera: Cucujidae). |
| PubMed: | Chemotaxonomic study of undescribed species ofMyrmica ant from Idaho. |
| PubMed: | Induction of chromosome loss by mixtures of organic solvents including neurotoxins. |
| PubMed: | Odor volatiles associated with microflora in damp ventilated and non-ventilated bin-stored bulk wheat. |
| PubMed: | Saw-toothed grain beetleOryzaephilus surinamensis (L.) (Coleoptera: Silvanidae) : Collection, identification, and bioassay of attractive volatiles from beetles and oats. |
| PubMed: | Fungal volatiles associated with moldy grain in ventilated and non-ventilated bin-stored wheat. |
| PubMed: | Sensitivity to sunscreens. |
| PubMed: | The size of mitral cells is altered when rats are exposed to an odor from their day of birth. |
| PubMed: | Electroantennogram responses of grape borerXylotrechus pyrrhoderus bates (Coleoptera: Cerambycidae) to its male sex pheromone components. |
| PubMed: | Experimental study on the enhancement of the neurotoxicity of methyl n-butyl ketone by non-neurotoxic aliphatic monoketones. |
| PubMed: | Volatile constituents of Trichothecium roseum. |
| PubMed: | Sensitivity to isopropyl alcohol. |
| PubMed: | Studies on mushroom flavours 2. Flavour compounds in coprinus comatus. |
| PubMed: | Identification, role and systematic significance of 3-octanone in the carpenter ant, Camponotus schaefferi WHR. |
| PubMed: | Volatile Flavor Compounds Produced by Molds of Aspergillus, Penicillium, and Fungi imperfecti. |
| PubMed: | Identification of the predominant volatile compounds produced by Aspergillus flavus. |
| PubMed: | [Colorimetric determination of 3-octanone using 3, 5-dinitrobenzoic acid]. |
|
 3D/inchi
3D/inchi




















