Articles:
Notes:
None found
| CAS Number: | 53963-43-2 | 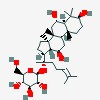 3D/inchi 3D/inchi
|
| Other(deleted CASRN): | 156912-00-4 | |
| Nikkaji Web: | J38.859I | |
| MDL: | MFCD06410947 | |
| XlogP3-AA: | 4.30 (est) | |
| Molecular Weight: | 638.88294000 | |
| Formula: | C36 H62 O9 | |
| NMR Predictor: | Predict (works with chrome or firefox) | |
Category: natural substances and extractives
US / EU / FDA / JECFA / FEMA / FLAVIS / Scholar / Patent Information:
| Google Scholar: | Search |
| Google Books: | Search |
| Google Scholar: with word "volatile" | Search |
| Google Scholar: with word "flavor" | Search |
| Google Scholar: with word "odor" | Search |
| Google Patents: | Search |
| US Patents: | Search |
| EU Patents: | Search |
| Pubchem Patents: | Search |
| PubMed: | Search |
| NCBI: | Search |
Physical Properties:
| Assay: | 95.00 to 100.00 % |
| Food Chemicals Codex Listed: | No |
| Soluble in: | |
| water, 0.07331 mg/L @ 25 °C (est) | |
Organoleptic Properties:
| Odor and/or flavor descriptions from others (if found). | |
Cosmetic Information:
| None found |
Suppliers:
| Alfa Biotechnology |
| For experimental / research use only. |
| Ginsenoside F1 98% |
| BOC Sciences |
| For experimental / research use only. |
| Ginsenoside F1 |
| Carbosynth |
| For experimental / research use only. |
| Ginsenoside F1 |
| Sigma-Aldrich: Sigma |
| For experimental / research use only. |
| Ginsenoside F1 ≥98% (HPLC) |
Safety Information:
| Preferred SDS: View | |
| Hazards identification | |
| Classification of the substance or mixture | |
| GHS Classification in accordance with 29 CFR 1910 (OSHA HCS) | |
| None found. | |
| GHS Label elements, including precautionary statements | |
| Pictogram | |
| Hazard statement(s) | |
| None found. | |
| Precautionary statement(s) | |
| None found. | |
| Oral/Parenteral Toxicity: | |
| Not determined | |
| Dermal Toxicity: | |
| Not determined | |
| Inhalation Toxicity: | |
| Not determined | |
Safety in Use Information:
| Category: | natural substances and extractives | ||
| Recommendation for ginsenoside F1 usage levels up to: | |||
| not for fragrance use. | |||
| Recommendation for ginsenoside F1 flavor usage levels up to: | |||
| not for flavor use. | |||
Safety References:
| EPI System: | View |
| Cancer Citations: | Search |
| Toxicology Citations: | Search |
| EPA ACToR: | Toxicology Data |
| EPA Substance Registry Services (SRS): | Registry |
| Laboratory Chemical Safety Summary : | 9809542 |
| National Institute of Allergy and Infectious Diseases: | Data |
| WGK Germany: | 3 |
| (2R,3S,4S,5R,6S)-2-(hydroxymethyl)-6-[(2S)-6-methyl-2-[(3S,5R,6S,8R,9R,10R,12R,13R,14R,17S)-3,6,12-trihydroxy-4,4,8,10,14-pentamethyl-2,3,5,6,7,9,11,12,13,15,16,17-dodecahydro-1H-cyclopenta[a]phenanthren-17-yl]hept-5-en-2-yl]oxyoxane-3,4,5-triol | |
| Chemidplus: | 0053963432 |
References:
| (2R,3S,4S,5R,6S)-2-(hydroxymethyl)-6-[(2S)-6-methyl-2-[(3S,5R,6S,8R,9R,10R,12R,13R,14R,17S)-3,6,12-trihydroxy-4,4,8,10,14-pentamethyl-2,3,5,6,7,9,11,12,13,15,16,17-dodecahydro-1H-cyclopenta[a]phenanthren-17-yl]hept-5-en-2-yl]oxyoxane-3,4,5-triol | |
| NIST Chemistry WebBook: | Search Inchi |
| Pubchem (cid): | 9809542 |
| Pubchem (cas): | 53963-43-2 |
Other Information:
| (IUPAC): | Atomic Weights of the Elements 2011 (pdf) |
| Videos: | The Periodic Table of Videos |
| tgsc: | Atomic Weights use for this web site |
| (IUPAC): | Periodic Table of the Elements |
| CHEBI: | View |
| CHEMBL: | View |
| KEGG (GenomeNet): | C20780 |
| HMDB (The Human Metabolome Database): | HMDB39555 |
| FooDB: | FDB019176 |
| VCF-Online: | VCF Volatile Compounds in Food |
| ChemSpider: | View |
Potential Blenders and core components note
| None Found | ||
Potential Uses:
| None Found |
Occurrence (nature, food, other): note
| panax ginseng Search Trop Picture |
Synonyms:
| beta-D- | glucopyranoside, (3beta,6aalpha,12beta)-3,6,12-trihydroxydammar-24-ene-20-yl |
| (2R,3S,4S,5R,6S)-2-( | hydroxymethyl)-6-[(2S)-6-methyl-2-[(3S,5R,6S,8R,9R,10R,12R,13R,14R,17S)-3,6,12-trihydroxy-4,4,8,10,14-pentamethyl-2,3,5,6,7,9,11,12,13,15,16,17-dodecahydro-1H-cyclopenta[a]phenanthren-17-yl]hept-5-en-2-yl]oxyoxane-3,4,5-triol |
