Articles:
4-hydroxyphenyl a-D-glucopyranoside
Notes:
None found
| CAS Number: | 84380-01-8 | 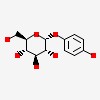 3D/inchi 3D/inchi
|
| FDA UNII: | 72VUP07IT5 | |
| Nikkaji Web: | J593.405B | |
| Beilstein Number: | 89675 | |
| MDL: | MFCD09838262 | |
| XlogP3-AA: | -0.70 (est) | |
| Molecular Weight: | 272.25392000 | |
| Formula: | C12 H16 O7 | |
| NMR Predictor: | Predict (works with chrome or firefox) | |
Category: antioxidant, bleaching, skin conditioning
US / EU / FDA / JECFA / FEMA / FLAVIS / Scholar / Patent Information:
| Google Scholar: | Search |
| Google Books: | Search |
| Google Scholar: with word "volatile" | Search |
| Google Scholar: with word "flavor" | Search |
| Google Scholar: with word "odor" | Search |
| Google Patents: | Search |
| US Patents: | Search |
| EU Patents: | Search |
| Pubchem Patents: | Search |
Physical Properties:
| Assay: | 95.00 to 100.00 % |
| Food Chemicals Codex Listed: | No |
| Soluble in: | |
| water, 9.591e+005 mg/L @ 25 °C (est) | |
Organoleptic Properties:
| Odor and/or flavor descriptions from others (if found). | |
Cosmetic Information:
| CosIng: | cosmetic data |
| Cosmetic Uses: |
antioxidants bleaching agents skin conditioning |
Suppliers:
| Alfa Biotechnology |
| For experimental / research use only. |
| a-Arbutin 98% |
| BOC Sciences |
| For experimental / research use only. |
| alpha-Arbutin |
| ECSA Chemicals |
| ALPHA ARBUTIN |
| ECSA TRADE THE MOST UPDATED FINANCIAL PUBLICATION ON THE WORLD OF CHEMISTRY |
| Glentham Life Sciences |
| alpha-Arbutin |
| Jiangyin Healthway |
| Alpha-arbutin |
| New functional food ingredients |
| M.C.Biotec |
| alpha-Arbutin |
| Meafo |
| For experimental / research use only. |
| Alpha-arbutin 99% |
| Shaanxi Y-Herb Biotechnology |
| Pure Alpha Arbutin 99% Bearberry Extract Powder For Skin Whiting CAS 84380-01-8 |
| Sigma-Aldrich |
| For experimental / research use only. |
| a-Arbutin analytical standard |
| Spec-Chem Industry |
| SpecWhite ® 03(A-arbutin) |
Safety Information:
| Hazards identification | |
| Classification of the substance or mixture | |
| GHS Classification in accordance with 29 CFR 1910 (OSHA HCS) | |
| None found. | |
| GHS Label elements, including precautionary statements | |
| Pictogram | |
| Hazard statement(s) | |
| None found. | |
| Precautionary statement(s) | |
| None found. | |
| Oral/Parenteral Toxicity: | |
| Not determined | |
| Dermal Toxicity: | |
| Not determined | |
| Inhalation Toxicity: | |
| Not determined | |
Safety in Use Information:
| Category: | antioxidant, bleaching, skin conditioning | ||
| Recommendation for alpha-arbutin usage levels up to: | |||
| not for fragrance use. | |||
| Recommendation for alpha-arbutin flavor usage levels up to: | |||
| not for flavor use. | |||
Safety References:
| EPI System: | View |
| EPA ACToR: | Toxicology Data |
| EPA Substance Registry Services (SRS): | Registry |
| Laboratory Chemical Safety Summary : | 158637 |
| National Institute of Allergy and Infectious Diseases: | Data |
| WGK Germany: | 3 |
| (2R,3S,4S,5R,6R)-2-(hydroxymethyl)-6-(4-hydroxyphenoxy)oxane-3,4,5-triol | |
| Chemidplus: | 0084380018 |
References:
| (2R,3S,4S,5R,6R)-2-(hydroxymethyl)-6-(4-hydroxyphenoxy)oxane-3,4,5-triol | |
| NIST Chemistry WebBook: | Search Inchi |
| Pubchem (cid): | 158637 |
| Pubchem (sid): | 135114345 |
Other Information:
| (IUPAC): | Atomic Weights of the Elements 2011 (pdf) |
| Videos: | The Periodic Table of Videos |
| tgsc: | Atomic Weights use for this web site |
| (IUPAC): | Periodic Table of the Elements |
| CHEBI: | View |
| CHEMBL: | View |
| KEGG (GenomeNet): | C12079 |
| HMDB (The Human Metabolome Database): | Search |
| ChemSpider: | View |
| Wikipedia: | View |
Potential Blenders and core components note
| None Found | ||
Potential Uses:
| None Found |
Occurrence (nature, food, other): note
| not found in nature |
Synonyms:
| a-D- | glucopyranoside, 4-hydroxyphenyl |
| (2R,3S,4S,5R,6R)-2-( | hydroxymethyl)-6-(4-hydroxyphenoxy)oxane-3,4,5-triol |
| 4- | hydroxyphenyl a-D-glucopyranoside |
| 4- | hydroxyphenyl-alpha-D-glucopyranoside |



