Articles:
(17R,21-alpha)-ajmalan-17,21-diol
Notes:
an alkaloid found in the root of rauwolfia serpentina, among other plant sources. it is a class ia antiarrhythmic agent that apparently acts by changing the shape and threshold of cardiac action potentials.
| CAS Number: | 4360-12-7 | 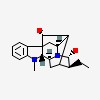 3D/inchi 3D/inchi
|
| ECHA EINECS - REACH Pre-Reg: | 224-439-4 | |
| FDA UNII: | 1PON08459R | |
| MDL: | MFCD00135652 | |
| XlogP3: | 1.80 (est) | |
| Molecular Weight: | 326.43962000 | |
| Formula: | C20 H26 N2 O2 | |
| NMR Predictor: | Predict (works with chrome or firefox) | |
Category: natural substances and extractives
US / EU / FDA / JECFA / FEMA / FLAVIS / Scholar / Patent Information:
| Google Scholar: | Search |
| Google Books: | Search |
| Google Scholar: with word "volatile" | Search |
| Google Scholar: with word "flavor" | Search |
| Google Scholar: with word "odor" | Search |
| Google Patents: | Search |
| US Patents: | Search |
| EU Patents: | Search |
| Pubchem Patents: | Search |
| PubMed: | Search |
| NCBI: | Search |
Physical Properties:
| Assay: | 95.00 to 100.00 % |
| Food Chemicals Codex Listed: | No |
| Melting Point: | 206.00 °C. @ 760.00 mm Hg |
| Boiling Point: | 519.40 °C. @ 760.00 mm Hg (est) |
| Flash Point: | 545.00 °F. TCC ( 285.20 °C. ) (est) |
| logP (o/w): | 1.810 |
| Soluble in: | |
| water, 490 mg/L @ 30 °C (exp) | |
| water, 2486 mg/L @ 25 °C (est) | |
Organoleptic Properties:
| Odor and/or flavor descriptions from others (if found). | |
Cosmetic Information:
| None found |
Suppliers:
| BOC Sciences |
| For experimental / research use only. |
| Ajmaline:Ajmalan-17,21-diol,(17R,21)-, 95%
Odor: characteristic Use: Ajmaline is an alkaloid which could extracted from Rauwolfia serpentina. It is an antiarrhythmic agent, acting through changing the shreshold and the shape of cardiac action potentials.
Ajmaline is an antiarrhythmic agent acting through changing the shreshold and the shape of cardiac action potentials |
| Carbosynth |
| For experimental / research use only. |
| Ajmaline |
| ExtraSynthese |
| For experimental / research use only. |
| Ajmaline (HPLC) ≥90% |
| Sigma-Aldrich: Sigma |
| For experimental / research use only. |
| Ajmaline |
Safety Information:
| Preferred SDS: View | |
| Hazards identification | |
| Classification of the substance or mixture | |
| GHS Classification in accordance with 29 CFR 1910 (OSHA HCS) | |
| None found. | |
| GHS Label elements, including precautionary statements | |
| Pictogram | |
| Hazard statement(s) | |
| None found. | |
| Precautionary statement(s) | |
| None found. | |
| Oral/Parenteral Toxicity: | |
|
oral-bird - wild LD50 178 mg/kg Archives of Environmental Contamination and Toxicology. Vol. 12, Pg. 355, 1983. oral-child LDLo 53125 ug/kg GASTROINTESTINAL: "HYPERMOTILITY, DIARRHEA" GASTROINTESTINAL: NAUSEA OR VOMITING LUNGS, THORAX, OR RESPIRATION: ACUTE PULMONARY EDEMA Journal of Forensic Sciences. Vol. 33, Pg. 558, 1988. intravenous-guinea pig LDLo 28 mg/kg CARDIAC: CHANGE IN RATE Farmaco, Edizione Scientifica. Vol. 19, Pg. 865, 1964. oral-man TDLo 32 mg/kg CARDIAC: EKG CHANGES NOT DIAGNOSTIC OF ABOVE BEHAVIORAL: GENERAL ANESTHETIC LUNGS, THORAX, OR RESPIRATION: CYANOSIS American Heart Journal. Vol. 70, Pg. 719, 1965. intraperitoneal-mouse LD50 75 mg/kg National Technical Information Service. Vol. AD691-490 intravenous-mouse LD50 21 mg/kg European Journal of Toxicology and Environmental Hygiene. Vol. 8, Pg. 188, 1975. oral-mouse LD50 255 mg/kg CARDIAC: CHANGE IN RATE Farmaco, Edizione Scientifica. Vol. 19, Pg. 865, 1964. unreported-mouse LD50 220 mg/kg Pharmaceutical Chemistry Journal Vol. 21, Pg. 331, 1987. oral-quail LDLo 316 mg/kg Ecotoxicology and Environmental Safety. Vol. 6, Pg. 149, 1982. intraperitoneal-rat LD50 94 mg/kg Pharmaceutical Chemistry Journal Vol. 21, Pg. 331, 1987. intravenous-rat LD50 26 mg/kg CARDIAC: OTHER CHANGES Pharmazie. Vol. 31, Pg. 36, 1976. oral-rat LD50 360 mg/kg BEHAVIORAL: ATAXIA BEHAVIORAL: CONVULSIONS OR EFFECT ON SEIZURE THRESHOLD BEHAVIORAL: ALTERED SLEEP TIME (INCLUDING CHANGE IN RIGHTING REFLEX) Pharmazie. Vol. 31, Pg. 39, 1976. | |
| Dermal Toxicity: | |
|
subcutaneous-mouse LD50 180 mg/kg CARDIAC: CHANGE IN RATE Farmaco, Edizione Scientifica. Vol. 19, Pg. 865, 1964. subcutaneous-rat LD50 216 mg/kg CARDIAC: CHANGE IN RATE Farmaco, Edizione Scientifica. Vol. 19, Pg. 865, 1964. | |
| Inhalation Toxicity: | |
| Not determined | |
Safety in Use Information:
| Category: | natural substances and extractives | ||
| Recommendation for ajmaline usage levels up to: | |||
| not for fragrance use. | |||
| Recommendation for ajmaline flavor usage levels up to: | |||
| not for flavor use. | |||
Safety References:
| EPI System: | View |
| ClinicalTrials.gov: | search |
| AIDS Citations: | Search |
| Cancer Citations: | Search |
| Toxicology Citations: | Search |
| EPA ACToR: | Toxicology Data |
| EPA Substance Registry Services (SRS): | Registry |
| Laboratory Chemical Safety Summary : | 174236 |
| National Institute of Allergy and Infectious Diseases: | Data |
| WGK Germany: | 3 |
| Chemidplus: | 0004360127 |
| RTECS: | AX8050000 for cas# 4360-12-7 |
References:
| NIST Chemistry WebBook: | Search Inchi |
| Pubchem (cid): | 174236 |
| Pubchem (sid): | 134985550 |
Other Information:
| (IUPAC): | Atomic Weights of the Elements 2011 (pdf) |
| Videos: | The Periodic Table of Videos |
| tgsc: | Atomic Weights use for this web site |
| (IUPAC): | Periodic Table of the Elements |
| CHEBI: | View |
| HMDB (The Human Metabolome Database): | HMDB15495 |
| VCF-Online: | VCF Volatile Compounds in Food |
| ChemSpider: | View |
| Wikipedia: | View |
Potential Blenders and core components note
| None Found | ||
Potential Uses:
| None Found |
Occurrence (nature, food, other): note
| rauwolfia serpentina root Search Trop Picture |
Synonyms:
| ajmalan-17,21-diol | |
| (17R,21-alpha)- | ajmalan-17,21-diol |
| (17R,21alpha)- | ajmalan-17,21-diol |
| ajmalan-17,21-diol, (17R,21-alpha)- (9CI) | |
| ajmalan-17,21-diol, (17R,21alpha)-, compound with methanol (1:1) | |
| ajmalin | |
| aritmina | |
| cardiorythmine | |
| cartagine | |
| gilurytmal | |
| ignazin | |
| merabitol | |
| raugalline | |
| rauwolfin | |
| rauwolfine | |
| rhytmaton | |
| ritmos | |
| rytmalin | |
| siddiqui | |
| tachmalin | |
| tajmalin | |
| takhmalin | |
| takycor |
