Articles:
adenine, 9b-D-arabinofuranosyl-
Notes:
a nucleoside antibiotic isolated from streptomyces antibioticus. it has some antineoplastic properties and has broad spectrum activity against dna viruses in cell cultures and significant antiviral activity against infections caused by a variety of viruses such as the herpes viruses, the vaccinia virus and varicella zoster virus.
| CAS Number: | 5536-17-4 | 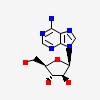 3D/inchi 3D/inchi
|
| ECHA EINECS - REACH Pre-Reg: | 226-893-9 | |
| FDA UNII: | 3XQD2MEW34 | |
| Nikkaji Web: | J3.415K | |
| Beilstein Number: | 0624881 | |
| MDL: | MFCD00065471 | |
| XlogP3: | -1.10 (est) | |
| Molecular Weight: | 267.24541000 | |
| Formula: | C10 H13 N5 O4 | |
| BioActivity Summary: | listing | |
| NMR Predictor: | Predict (works with chrome or firefox) | |
Category: pharmaceuticals / chemical synthisis
US / EU / FDA / JECFA / FEMA / FLAVIS / Scholar / Patent Information:
| Google Scholar: | Search |
| Google Books: | Search |
| Google Scholar: with word "volatile" | Search |
| Google Scholar: with word "flavor" | Search |
| Google Scholar: with word "odor" | Search |
| Google Patents: | Search |
| US Patents: | Search |
| EU Patents: | Search |
| Pubchem Patents: | Search |
| PubMed: | Search |
| NCBI: | Search |
Physical Properties:
| Appearance: | white crystalline powder (est) |
| Assay: | 95.00 to 100.00 % |
| Food Chemicals Codex Listed: | No |
| Melting Point: | 257.00 °C. @ 760.00 mm Hg |
| Boiling Point: | 676.27 °C. @ 760.00 mm Hg (est) |
| Vapor Pressure: | 6.000000 mmHg @ 25.00 °C. (est) |
| Flash Point: | 685.00 °F. TCC ( 362.80 °C. ) (est) |
| logP (o/w): | -0.755 (est) |
| Soluble in: | |
| water, 8228 mg/L @ 25 °C (est) | |
Organoleptic Properties:
| Odor and/or flavor descriptions from others (if found). | |
Cosmetic Information:
| None found |
Suppliers:
| BOC Sciences |
| For experimental / research use only. |
| Adenine 9-b-D-arabinofuranoside (Vidarabine) > 99%
Odor: characteristic Use: Arabinofuranosyl-adenine (ara-A) is an analog of adenosine with an antiviral property. It is often used as a prodrug that can be both substrate and inhibitor of DNA polymerase. |
| Carbosynth |
| For experimental / research use only. |
| 9-(b-D-Arabinofuranosyl)adenine |
| George Uhe Company |
| 9-B-D-Arabinofuranosyladenine Monohydrate |
| Glentham Life Sciences |
| Adenine 9-beta-D-arabinofuranoside |
| Santa Cruz Biotechnology |
| For experimental / research use only. |
| Vidarabine |
| Sigma-Aldrich: Sigma |
| For experimental / research use only. |
| Adenine 9-b-D-arabinofuranoside ≥99% |
Safety Information:
| Preferred SDS: View | |
| Hazards identification | |
| Classification of the substance or mixture | |
| GHS Classification in accordance with 29 CFR 1910 (OSHA HCS) | |
| None found. | |
| GHS Label elements, including precautionary statements | |
| Pictogram | |
| Hazard statement(s) | |
| None found. | |
| Precautionary statement(s) | |
| None found. | |
| Oral/Parenteral Toxicity: | |
|
intravenous-human TDLo 300 ug/kg GASTROINTESTINAL: "HYPERMOTILITY, DIARRHEA" BEHAVIORAL: ANOREXIA (HUMAN Journal of Infectious Diseases. Vol. 133, Pg. A192, 1976. intravenous-human TDLo 2 mg/kg BEHAVIORAL: ATAXIA BRAIN AND COVERINGS: CHANGES IN SURFACE EEG BEHAVIORAL: TOXIC PSYCHOSIS Journal of Infectious Diseases. Vol. 134, Pg. 75, 1976. intraperitoneal-mouse LD50 3057 mg/kg Iyakuhin Kenkyu. Study of Medical Supplies. Vol. 15, Pg. 688, 1984. intravenous-mouse LD50 442 mg/kg Iyakuhin Kenkyu. Study of Medical Supplies. Vol. 15, Pg. 688, 1984. oral-mouse LD50 7800 ug/kg "Kirk-Othmer Encyclopedia of Chemical Technology," 3rd ed., Grayson, M., and D. Eckroth, eds. New York, John Wiley & Sons, Inc., 1978Vol. 2, Pg. 962, 1978. intraperitoneal-rat LD50 1476 mg/kg Iyakuhin Kenkyu. Study of Medical Supplies. Vol. 15, Pg. 688, 1984. intravenous-rat LD50 302 mg/kg Iyakuhin Kenkyu. Study of Medical Supplies. Vol. 15, Pg. 688, 1984. oral-rat LD50 > 5000 mg/kg LIVER: OTHER CHANGES Annals of the New York Academy of Sciences. Vol. 284, Pg. 6, 1977. | |
| Dermal Toxicity: | |
|
subcutaneous-mouse LD50 5086 mg/kg Iyakuhin Kenkyu. Study of Medical Supplies. Vol. 15, Pg. 688, 1984. subcutaneous-rat LD50 8914 mg/kg Iyakuhin Kenkyu. Study of Medical Supplies. Vol. 15, Pg. 688, 1984. | |
| Inhalation Toxicity: | |
| Not determined | |
Safety in Use Information:
| Category: | pharmaceuticals / chemical synthisis | ||
| Recommendation for vidarabine usage levels up to: | |||
| not for fragrance use. | |||
| Recommendation for vidarabine flavor usage levels up to: | |||
| not for flavor use. | |||
Safety References:
| EPI System: | View |
| ClinicalTrials.gov: | search |
| Daily Med: | search |
| Chemical Carcinogenesis Research Information System: | Search |
| AIDS Citations: | Search |
| Cancer Citations: | Search |
| Toxicology Citations: | Search |
| EPA GENetic TOXicology: | Search |
| EPA Substance Registry Services (TSCA): | 5536-17-4 |
| EPA ACToR: | Toxicology Data |
| EPA Substance Registry Services (SRS): | Registry |
| Laboratory Chemical Safety Summary : | 21704 |
| National Institute of Allergy and Infectious Diseases: | Data |
| (2R,3S,4S,5R)-2-(6-aminopurin-9-yl)-5-(hydroxymethyl)oxolane-3,4-diol | |
| Chemidplus: | 0005536174 |
| EPA/NOAA CAMEO: | hazardous materials |
References:
| (2R,3S,4S,5R)-2-(6-aminopurin-9-yl)-5-(hydroxymethyl)oxolane-3,4-diol | |
| NIST Chemistry WebBook: | Search Inchi |
| Pubchem (cid): | 21704 |
| Pubchem (sid): | 134987129 |
Other Information:
| (IUPAC): | Atomic Weights of the Elements 2011 (pdf) |
| Videos: | The Periodic Table of Videos |
| tgsc: | Atomic Weights use for this web site |
| (IUPAC): | Periodic Table of the Elements |
| CHEBI: | View |
| CHEMBL: | View |
| KEGG (GenomeNet): | D06298 |
| HMDB (The Human Metabolome Database): | Search |
| MedlinePlusSupp: | View |
| ChemSpider: | View |
| Wikipedia: | View |
| EFSA Update of results on the monitoring of furan levels in food: | Read Report |
| EFSA Previous report: Results on the monitoring of furan levels in food: | Read Report |
| EFSA Report of the CONTAM Panel on provisional findings on furan in food: | Read Report |
| Formulations/Preparations: •ophthalmic ointment 3% (equivalent to vidarabine, vira-a, parke-davis anhydrous 2.8%) •parenteral: concentrate, for injection, for iv infusion, 200 mgml (equivalent to 187.4 mg of anhydrous vidarabine per ml). vira-a (with benzethonium chloride and phosphate buffers), parke-davis •vidarabine not currently approved for use in the united states | |
Potential Blenders and core components note
| None Found | ||
Potential Uses:
| None Found |
Occurrence (nature, food, other): note
| not found in nature |
Synonyms:
| adenine 9-b-D-arabinofuranoside | |
| adenine-9-b-D-arabinofuranoside | |
| adenine-b-D-arabinofuranoside | |
| adenine, 9-b-D-arabinofuranosyl- | |
| adenine, 9b-D-arabinofuranosyl- | |
| 6- | amino-9-b-D-arabinofuranosylpurine |
| (2R,3S,4S,5R)-2-(6- | amino-9H-purin-9-yl)-5-(hydroxymethyl)tetrahydrofuran-3,4-diol |
| (2R,3S,4S,5R)-2-(6- | aminopurin-9-yl)-5-(hydroxymethyl)oxolane-3,4-diol |
| 9-b-D- | arabinofuranosyl-9H-purin-6-amine (9CI) |
| 9-b-D- | arabinofuranosyl-9H-purine-6-amine |
| 9-b-D- | arabinofuranosyladenine |
| 9b-D- | arabinofuranosyladenine |
| (+)- | cyclaradine |
| spongoadenosine | |
| vidarabin | |
| vidarabinum |

