Articles:
(R)-1,6,6-trimethyl-1,2,6,7,8,9-hexahydro-phenanthro[1,2-b]furan-10,11-dione
Notes:
from salvia miltiorrhiza. Isol. from Rosmarinus officinalis (rosemary)
| CAS Number: | 35825-57-1 | 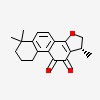 3D/inchi 3D/inchi
|
| FDA UNII: | 5E9SXT166N | |
| Nikkaji Web: | J865.486G | |
| MDL: | MFCD07636810 | |
| XlogP3-AA: | 3.80 (est) | |
| Molecular Weight: | 296.36600000 | |
| Formula: | C19 H20 O3 | |
| BioActivity Summary: | listing | |
| NMR Predictor: | Predict (works with chrome or firefox) | |
Category: natural substances and extractives
US / EU / FDA / JECFA / FEMA / FLAVIS / Scholar / Patent Information:
| Google Scholar: | Search |
| Google Books: | Search |
| Google Scholar: with word "volatile" | Search |
| Google Scholar: with word "flavor" | Search |
| Google Scholar: with word "odor" | Search |
| Google Patents: | Search |
| US Patents: | Search |
| EU Patents: | Search |
| Pubchem Patents: | Search |
| PubMed: | Search |
| NCBI: | Search |
Physical Properties:
| Appearance: | red-brown powder (est) |
| Assay: | 95.00 to 100.00 % |
| Food Chemicals Codex Listed: | No |
| Boiling Point: | 459.03 °C. @ 760.00 mm Hg (est) |
| Flash Point: | 398.00 °F. TCC ( 203.40 °C. ) (est) |
| logP (o/w): | 4.130 (est) |
| Soluble in: | |
| water, 0.5213 mg/L @ 25 °C (est) | |
Organoleptic Properties:
| Odor and/or flavor descriptions from others (if found). | |
Cosmetic Information:
| None found |
Suppliers:
| Alfa Biotechnology |
| For experimental / research use only. |
| Cryptotanshinone 98% |
| BOC Sciences |
| For experimental / research use only. |
| Cryptotanshinone
Odor: characteristic Use: Cryptotanshinone, a natural cell-permeable diterpene quinone isolated from Salvia miltiorrhiza, it inhibits acetylcholinesterase (IC50 = 4.09 mM) and reduces A peptide generation so that can treat Alzheimer's disease. It also inhibits STAT3 activity (IC
Implicated in Alzheimer's disease |
| Carbosynth |
| For experimental / research use only. |
| Cryptotanshinone |
| Coompo |
| For experimental / research use only. |
| Cryptotanshinone from Plants ≥96%
Odor: characteristic Use: Cryptotanshinone could have diverse effects on cell cycle events in melanoma cell lines with different metastatic capacity. This property might offer an opportunity to study underlying mechanisms for the different antitumor effects of administered Cryptotanshinone in B16 and B16BL6 cells.
Cryptotanshinone, the major active constituent isolated from the root of Salvia miltiorrhiza Bunge, has been recently evaluated for its anti-cancer activity, but the molecular mechanisms underlying these activities remain poorly understood. In particular, it remains completely unknown as to whether or not cryptotanshinone can induce endoplasmic reticulum (ER) stress. Herein, we identify cryptotanshinone as a potent stimulator of ER stress, leading to apoptosis in many cancer cell lines, including HepG2 hepatoma and MCF7 breast carcinoma, and also demonstrate that mitogen-activated protein kinases function as mediators in this process. Reactive oxygen species generated by cryptotanshinone have been shown to play a critical role in ER stress-induced apoptosis. Cryptotanshinone also evidenced sensitizing effects to a broad range of anti-cancer agents including Fas/Apo-1, TNF-a, cisplatin, etoposide or 5-FU through inducing ER stress, highlighting the therapeutic potential in the treatment of human hepatoma and breast cancer.
Displays antibacterial and anti-inflammatory activity and acts as an antidiabetes and antiobesity agent via activation of AMP-activated protein kinase (AMPK). Also improves cognitive impairment in Alzheimer's disease transgenic mice by inhibition of acetylcholinesterase (IC50 = 4.09 ÁM) and reduction in Aβ peptide generation. |
| Jiangyin Healthway |
| Cryptotanshinone |
| New functional food ingredients |
| Santa Cruz Biotechnology |
| For experimental / research use only. |
| Cryptotanshinone |
| Sigma-Aldrich: Sigma |
| For experimental / research use only. |
| Cryptotanshinone ≥98% (HPLC) |
| TCI AMERICA |
| For experimental / research use only. |
| Cryptotanshinone >97.0%(HPLC) |
Safety Information:
| Preferred SDS: View | |
| Hazards identification | |
| Classification of the substance or mixture | |
| GHS Classification in accordance with 29 CFR 1910 (OSHA HCS) | |
| None found. | |
| GHS Label elements, including precautionary statements | |
| Pictogram | |
| Hazard statement(s) | |
| None found. | |
| Precautionary statement(s) | |
| None found. | |
| Oral/Parenteral Toxicity: | |
| Not determined | |
| Dermal Toxicity: | |
| Not determined | |
| Inhalation Toxicity: | |
| Not determined | |
Safety in Use Information:
| Category: | natural substances and extractives | ||
| Recommendation for cryptotanshinone usage levels up to: | |||
| not for fragrance use. | |||
| Recommendation for cryptotanshinone flavor usage levels up to: | |||
| not for flavor use. | |||
Safety References:
| EPI System: | View |
| ClinicalTrials.gov: | search |
| Cancer Citations: | Search |
| Toxicology Citations: | Search |
| EPA ACToR: | Toxicology Data |
| EPA Substance Registry Services (SRS): | Registry |
| Laboratory Chemical Safety Summary : | 160254 |
| National Institute of Allergy and Infectious Diseases: | Data |
| WGK Germany: | 3 |
| (1R)-1,6,6-trimethyl-2,7,8,9-tetrahydro-1H-naphtho[1,2-g][1]benzofuran-10,11-dione | |
| Chemidplus: | 0035825571 |
References:
| (1R)-1,6,6-trimethyl-2,7,8,9-tetrahydro-1H-naphtho[1,2-g][1]benzofuran-10,11-dione | |
| NIST Chemistry WebBook: | Search Inchi |
| Pubchem (cid): | 160254 |
| Pubchem (sid): | 135115662 |
Other Information:
| (IUPAC): | Atomic Weights of the Elements 2011 (pdf) |
| Videos: | The Periodic Table of Videos |
| tgsc: | Atomic Weights use for this web site |
| (IUPAC): | Periodic Table of the Elements |
| CHEMBL: | View |
| HMDB (The Human Metabolome Database): | HMDB35220 |
| FooDB: | FDB013869 |
| Export Tariff Code: | 2932.99.6100 |
| VCF-Online: | VCF Volatile Compounds in Food |
| ChemSpider: | View |
| Wikipedia: | View |
| EFSA Update of results on the monitoring of furan levels in food: | Read Report |
| EFSA Previous report: Results on the monitoring of furan levels in food: | Read Report |
| EFSA Report of the CONTAM Panel on provisional findings on furan in food: | Read Report |
Potential Blenders and core components note
| None Found | ||
Potential Uses:
| None Found |
Occurrence (nature, food, other): note
| rosemary Search Trop Picture | |
| sage red sage Search Trop Picture |
Synonyms:
| (-)- | cryptotanshinone |
| (R)-1,2,6,7,8,9- | hexahydro-1,6,6-trimethyl-phenanthro(1,2-b)furan-10,11-dione |
| phenanthro(1,2-b)furan-10,11-dione, 1,2,6,7,8,9-hexahydro-1,6,6-trimethyl-, (R)- | |
| phenanthro[1,2-b]furan-10,11-dione, 1,2,6,7,8,9-hexahydro-1,6,6-trimethyl-, (1R)- | |
| tanshinone C | |
| (R)-1,6,6- | trimethyl-1,2,6,7,8,9-hexahydro-phenanthro[1,2-b]furan-10,11-dione |
| (1R)-1,6,6- | trimethyl-1,2,6,7,8,9-hexahydrophenanthro[1,2-b]furan-10,11-dione |
| (R)-1,6,6- | trimethyl-1,2,6,7,8,9-hexahydrophenanthro[1,2-b]furan-10,11-dione |
| (1R)-1,6,6- | trimethyl-2,7,8,9-tetrahydro-1H-naphtho[1,2-g][1]benzofuran-10,11-dione |


