Articles:
phenanthro[1,2-b]furan-10,11-dione, 1,6-dimethyl-
Notes:
None found
| CAS Number: | 568-73-0 | 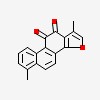 3D/inchi 3D/inchi
|
| FDA UNII: | 03UUH3J385 | |
| Nikkaji Web: | J12.294G | |
| MDL: | MFCD00210563 | |
| XlogP3-AA: | 3.70 (est) | |
| Molecular Weight: | 276.29124000 | |
| Formula: | C18 H12 O3 | |
| BioActivity Summary: | listing | |
| NMR Predictor: | Predict (works with chrome or firefox) | |
Category: pharmaceuticals / chemical synthisis
US / EU / FDA / JECFA / FEMA / FLAVIS / Scholar / Patent Information:
| Google Scholar: | Search |
| Google Books: | Search |
| Google Scholar: with word "volatile" | Search |
| Google Scholar: with word "flavor" | Search |
| Google Scholar: with word "odor" | Search |
| Google Patents: | Search |
| US Patents: | Search |
| EU Patents: | Search |
| Pubchem Patents: | Search |
| PubMed: | Search |
| NCBI: | Search |
Physical Properties:
| Appearance: | red-brown powder (est) |
| Assay: | 95.00 to 100.00 % |
| Food Chemicals Codex Listed: | No |
| Boiling Point: | 497.96 °C. @ 760.00 mm Hg (est) |
| Flash Point: | 475.00 °F. TCC ( 245.90 °C. ) (est) |
| logP (o/w): | 4.312 (est) |
| Soluble in: | |
| water, 0.04758 mg/L @ 25 °C (est) | |
Organoleptic Properties:
| Odor and/or flavor descriptions from others (if found). | |
Cosmetic Information:
| None found |
Suppliers:
| Alfa Biotechnology |
| For experimental / research use only. |
| Tanshinone I 98% |
| BOC Sciences |
| For experimental / research use only. |
| Tanshinone I 0.96
Odor: characteristic Use: Tanshinone I isolated from the roots of Salvia miltiorrhiza. It can protect against peroxynitrite-induced DNA damage, and hydroxyl radical formation and cytotoxicity, might have implications for Tanshinone I-mediated neuroprotection.
anti-tumor; anti-cancer; |
| Coompo |
| For experimental / research use only. |
| Tanshinone I from Plants ≥96%
Odor: characteristic Use: Tanshinone I significantly inhibited migration, invasion, and gelatinase activity in macrophage-conditioned medium-stimulated CL1-5 cells in vitro and also reduced the tumorigenesis and metastasis in CL1-5-bearing severe combined immunodeficient mice.
Tanshinone I possesses the strongest inhibitory effect on tumor necrosis factor-a (TNF-a)- induced adhesion molecules. Its potential anticoagulant effect should be noted and evaluated. |
| Santa Cruz Biotechnology |
| For experimental / research use only. |
| Tanshinone I |
| Sigma-Aldrich: Sigma |
| For experimental / research use only. |
| Tanshinone I ≥97% (HPLC) |
Safety Information:
| Preferred SDS: View | |
| Hazards identification | |
| Classification of the substance or mixture | |
| GHS Classification in accordance with 29 CFR 1910 (OSHA HCS) | |
| None found. | |
| GHS Label elements, including precautionary statements | |
| Pictogram | |
| Hazard statement(s) | |
| None found. | |
| Precautionary statement(s) | |
| None found. | |
| Oral/Parenteral Toxicity: | |
| Not determined | |
| Dermal Toxicity: | |
| Not determined | |
| Inhalation Toxicity: | |
| Not determined | |
Safety in Use Information:
| Category: | pharmaceuticals / chemical synthisis | ||
| Recommendation for tanshinone usage levels up to: | |||
| not for fragrance use. | |||
| Recommendation for tanshinone flavor usage levels up to: | |||
| not for flavor use. | |||
Safety References:
| EPI System: | View |
| Cancer Citations: | Search |
| Toxicology Citations: | Search |
| EPA ACToR: | Toxicology Data |
| EPA Substance Registry Services (SRS): | Registry |
| Laboratory Chemical Safety Summary : | 114917 |
| National Institute of Allergy and Infectious Diseases: | Data |
| WGK Germany: | 3 |
| 1,6-dimethylnaphtho[1,2-g][1]benzofuran-10,11-dione | |
| Chemidplus: | 0000568730 |
References:
| 1,6-dimethylnaphtho[1,2-g][1]benzofuran-10,11-dione | |
| NIST Chemistry WebBook: | Search Inchi |
| Pubchem (cid): | 114917 |
| Pubchem (sid): | 135071823 |
Other Information:
| (IUPAC): | Atomic Weights of the Elements 2011 (pdf) |
| Videos: | The Periodic Table of Videos |
| tgsc: | Atomic Weights use for this web site |
| (IUPAC): | Periodic Table of the Elements |
| CHEMBL: | View |
| HMDB (The Human Metabolome Database): | Search |
| ChemSpider: | View |
| EFSA Update of results on the monitoring of furan levels in food: | Read Report |
| EFSA Previous report: Results on the monitoring of furan levels in food: | Read Report |
| EFSA Report of the CONTAM Panel on provisional findings on furan in food: | Read Report |
Potential Blenders and core components note
| None Found | ||
Potential Uses:
| None Found |
Occurrence (nature, food, other): note
| not found in nature |
Synonyms:
| 1,6- | dimethyl-phenanthro[1,2-b]furan-10,11-dione |
| 1,6- | dimethylnaphtho[1,2-g][1]benzofuran-10,11-dione |
| 1,6- | dimethylphenanthro(1,2-b)furan-10,11-dione |
| 1,6- | dimethylphenanthro[1,2-b]furan-10,11-dione |
| phenanthro(1,2-b)furan-10,11-dione, 1,6-dimethyl- | |
| phenanthro[1,2-b]furan-10,11-dione, 1,6-dimethyl- | |
| tanshinon I | |
| tanshinone I | |
| tanshinone II B | |
| tanshinone IIB |
