Articles:
[1,3]benzodioxolo[5,6-c]-1,3-dioxolo[4,5-i]phenanthridinium, 13-methyl-
Notes:
bactericidal produces glaucoma in laboratory animals; from argemonemexicana & sanguinaria; inhibits glutamate decarboxylase. Consumption of Sanguinarine, present in poppy seeds and in the oil of Argemone mexicana which has been used as an adulterant for mustard oil in India, has been linked to development of glaucoma. Banned by FDA
Sanguinarine is a quaternary ammonium salt from the group of benzylisoquinoline alkaloids. It is extracted from some plants, including bloodroot (Sanguinaria canadensis), Mexican prickly poppy Argemone mexicana, Chelidonium majus and Macleaya cordata. It is also found in the root, stem and leaves of the opium poppy but not in the capsule.[citation needed]; Sanguinarine is a toxin that kills animal cells through its action on the Na+-K+-ATPase transmembrane protein. Epidemic dropsy is a disease that results from ingesting sanguinarine.
| CAS Number: | 2447-54-3 | 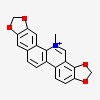 3D/inchi 3D/inchi
|
| FDA UNII: | AV9VK043SS | |
| Nikkaji Web: | J7.576K | |
| Beilstein Number: | 3915507 | |
| MDL: | MFCD00064925 | |
| XlogP3-AA: | 4.40 (est) | |
| Molecular Weight: | 332.33538000 | |
| Formula: | C20 H14 N O4 | |
| BioActivity Summary: | listing | |
| NMR Predictor: | Predict (works with chrome or firefox) | |
Category: pharmaceuticals / chemical synthisis
US / EU / FDA / JECFA / FEMA / FLAVIS / Scholar / Patent Information:
| Google Scholar: | Search |
| Google Books: | Search |
| Google Scholar: with word "volatile" | Search |
| Google Scholar: with word "flavor" | Search |
| Google Scholar: with word "odor" | Search |
| Google Patents: | Search |
| US Patents: | Search |
| EU Patents: | Search |
| Pubchem Patents: | Search |
| PubMed: | Search |
| NCBI: | Search |
Physical Properties:
| Appearance: | yellow-brown powder (est) |
| Assay: | 95.00 to 100.00 % |
| Food Chemicals Codex Listed: | No |
| Melting Point: | 266.00 °C. @ 760.00 mm Hg |
| Flash Point: | 32.00 °F. TCC ( 0.00 °C. ) (est) |
| logP (o/w): | -0.653 (est) |
| Soluble in: | |
| water, 3.316 mg/L @ 25 °C (est) | |
Organoleptic Properties:
| Odor and/or flavor descriptions from others (if found). | |
Cosmetic Information:
| None found |
Suppliers:
| Alfa Biotechnology |
| For experimental / research use only. |
| Sanguinarine 98% |
| BOC Sciences |
| For experimental / research use only. |
| Sanguinarine >98%
Odor: characteristic Use: Sanguinarine caused cell death in a dose dependent manner in all neuroblastoma cell lines except SK-N-BE(2) with rates of 18% in SH-SY5Y and 21% in Kelly human neuroblastoma cells. |
| Carbosynth |
| For experimental / research use only. |
| Sanguinarine |
| Changsha Organic Herb |
| Macleaya Cordata Extract |
Safety Information:
| Hazards identification | |
| Classification of the substance or mixture | |
| GHS Classification in accordance with 29 CFR 1910 (OSHA HCS) | |
| None found. | |
| GHS Label elements, including precautionary statements | |
| Pictogram | |
| Hazard statement(s) | |
| None found. | |
| Precautionary statement(s) | |
| None found. | |
| Oral/Parenteral Toxicity: | |
|
intraperitoneal-mouse LD50 18 mg/kg "CRC Handbook of Antibiotic Compounds," Vols.1- , Berdy, J., Boca Raton, FL, CRC Press, 1980Vol. 8(1), Pg. 195, 1982. intravenous-mouse LD50 19400 ug/kg BEHAVIORAL: CHANGES IN MOTOR ACTIVITY (SPECIFIC ASSAY) Farmakologiya i Toksikologiya Vol. 29, Pg. 76, 1966. intraperitoneal-rat LD50 18 mg/kg Indian Journal of Cancer. Vol. 5, Pg. 183, 1968. intravenous-rat LD50 28700 ug/kg Toxicologist. Vol. 5, Pg. 176, 1985. oral-rat LD50 1660 mg/kg Toxicologist. Vol. 5, Pg. 176, 1985. | |
| Dermal Toxicity: | |
|
subcutaneous-cat LD50 120 mg/kg Arzneimittel-Forschung. Drug Research. Vol. 10, Pg. 135, 1960. subcutaneous-dog LD50 70 mg/kg Arzneimittel-Forschung. Drug Research. Vol. 10, Pg. 135, 1960. subcutaneous-frog LD50 70 mg/kg Arzneimittel-Forschung. Drug Research. Vol. 10, Pg. 135, 1960. subcutaneous-mouse LD50 80 mg/kg Arzneimittel-Forschung. Drug Research. Vol. 10, Pg. 135, 1960. subcutaneous-rabbit LD50 125 mg/kg Arzneimittel-Forschung. Drug Research. Vol. 10, Pg. 135, 1960. | |
| Inhalation Toxicity: | |
|
inhalation-rat LC50 2200000 mg/m3 Toxicologist. Vol. 5, Pg. 176, 1985. | |
Safety in Use Information:
| Category: | pharmaceuticals / chemical synthisis | ||
| Recommendation for sanguinarine usage levels up to: | |||
| not for fragrance use. | |||
| Recommendation for sanguinarine flavor usage levels up to: | |||
| not for flavor use. | |||
Safety References:
| EPI System: | View |
| AIDS Citations: | Search |
| Cancer Citations: | Search |
| Toxicology Citations: | Search |
| EPA ACToR: | Toxicology Data |
| EPA Substance Registry Services (SRS): | Registry |
| Laboratory Chemical Safety Summary : | 5154 |
| National Institute of Allergy and Infectious Diseases: | Data |
| Chemidplus: | 0002447543 |
References:
| NIST Chemistry WebBook: | Search Inchi |
| Pubchem (cid): | 5154 |
| Pubchem (sid): | 134981925 |
Other Information:
| (IUPAC): | Atomic Weights of the Elements 2011 (pdf) |
| Videos: | The Periodic Table of Videos |
| tgsc: | Atomic Weights use for this web site |
| (IUPAC): | Periodic Table of the Elements |
| CHEBI: | View |
| CHEMBL: | View |
| KEGG (GenomeNet): | C06162 |
| HMDB (The Human Metabolome Database): | HMDB29367 |
| FooDB: | FDB000436 |
| VCF-Online: | VCF Volatile Compounds in Food |
| ChemSpider: | View |
| Wikipedia: | View |
Potential Blenders and core components note
| None Found | ||
Potential Uses:
| None Found |
Occurrence (nature, food, other): note
| argemone mexicana Search Trop Picture | |
| poppy opium poppy Search Trop Picture | |
| sanguinaria canadensis Search Trop Picture |
Synonyms:
| (1,3)- | benzodioxolo(5,6-c)-1,3-dioxolo(4,5-i)phenanthridinium, 13-methyl- |
| [1,3] | benzodioxolo[5,6-c]-1,3-dioxolo[4,5-i]phenanthridinium, 13-methyl- |
| dimethylenedioxy benzphenanthridine | |
| 13- | methyl-2H,10H-[1,3]dioxolo[4,5-i][1,3]dioxolo[4',5':4,5]benzo[1,2-c]phenanthridinium |
| 13- | methyl[1,3]benzodioxolo[5,6-c]-1,3-dioxolo[4,5-i]phenanthridinium |
| 13- | methyl[1,3]benzodioxolo[5,6-c][1,3]dioxolo[4,5-i]phenanthridin-13-ium |
| sanguinarin | |
| sanguiritrin | |
| sangvinarin | |
| veadent | |
| viadent |
