Articles:
4H-1-benzopyran-4-one, 3,7-dihydroxy-2-(3,4,5-trihydroxyphenyl)- (9CI)
Notes:
None found
| CAS Number: | 490-31-3 | 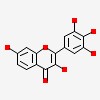 3D/inchi 3D/inchi
|
| ECHA EINECS - REACH Pre-Reg: | 207-709-6 | |
| FDA UNII: | KJ6DBC4U7E | |
| Nikkaji Web: | J1.551B | |
| Beilstein Number: | 0308905 | |
| MDL: | MFCD00016783 | |
| XlogP3-AA: | 1.60 (est) | |
| Molecular Weight: | 302.23910000 | |
| Formula: | C15 H10 O7 | |
| BioActivity Summary: | listing | |
| NMR Predictor: | Predict (works with chrome or firefox) | |
Category: natural substances and extractives
US / EU / FDA / JECFA / FEMA / FLAVIS / Scholar / Patent Information:
| Google Scholar: | Search |
| Google Books: | Search |
| Google Scholar: with word "volatile" | Search |
| Google Scholar: with word "flavor" | Search |
| Google Scholar: with word "odor" | Search |
| Google Patents: | Search |
| US Patents: | Search |
| EU Patents: | Search |
| Pubchem Patents: | Search |
| PubMed: | Search |
| NCBI: | Search |
Physical Properties:
| Assay: | 95.00 to 100.00 % |
| Food Chemicals Codex Listed: | No |
| Boiling Point: | 669.90 °C. @ 760.00 mm Hg (est) |
| Flash Point: | 497.00 °F. TCC ( 258.60 °C. ) (est) |
| logP (o/w): | 2.550 (est) |
| Soluble in: | |
| water, 4972 mg/L @ 25 °C (est) | |
Organoleptic Properties:
| Odor and/or flavor descriptions from others (if found). | |
Cosmetic Information:
| None found |
Suppliers:
| Alfa Biotechnology |
| For experimental / research use only. |
| Norkanugin 98% |
| BOC Sciences |
| For experimental / research use only. |
| ROBINETIN >98%
Odor: characteristic Use: Robinetin is from the leaves of Robinia pseudacacia. It inhibits EYPC membrane lipid peroxidation and HbA glycosylation with high efficiency. It could lead to the occurrence of positive induced circular dichroism (ICD) bands in the near ultra-violet (UV)
antioxidant/antiradical/anti-mutagenesis/anti-promotion |
| Carbosynth |
| For experimental / research use only. |
| Robinetin |
| Coompo |
| For experimental / research use only. |
| Robinetin from Plants ≥96%
Odor: characteristic Use: Antioxidant and antiradical activities.
Anti-mutagenesis and anti-promotion.
Incorporation of robinetin molecules in the chiral environment of the β-CDxs strongly affects the electronic transitions of robinetin leading to the occurrence of positive induced circular dichroism (ICD) bands in the near ultra-violet (UV) region. Molecular mechanics calculations show that the inclusion complex with the chromone ring inserted into the β-CDx cavity is most favorable, in agreement with our spectroscopic data. |
| ExtraSynthese |
| For experimental / research use only. |
| Robinetin (HPLC) ≥99% |
Safety Information:
| Hazards identification | |
| Classification of the substance or mixture | |
| GHS Classification in accordance with 29 CFR 1910 (OSHA HCS) | |
| None found. | |
| GHS Label elements, including precautionary statements | |
| Pictogram | |
| Hazard statement(s) | |
| None found. | |
| Precautionary statement(s) | |
| None found. | |
| Oral/Parenteral Toxicity: | |
| Not determined | |
| Dermal Toxicity: | |
| Not determined | |
| Inhalation Toxicity: | |
| Not determined | |
Safety in Use Information:
| Category: | natural substances and extractives | ||
| Recommendation for robinetin usage levels up to: | |||
| not for fragrance use. | |||
| Recommendation for robinetin flavor usage levels up to: | |||
| not for flavor use. | |||
Safety References:
| EPI System: | View |
| Chemical Carcinogenesis Research Information System: | Search |
| Cancer Citations: | Search |
| Toxicology Citations: | Search |
| EPA ACToR: | Toxicology Data |
| EPA Substance Registry Services (SRS): | Registry |
| Laboratory Chemical Safety Summary : | 5281692 |
| National Institute of Allergy and Infectious Diseases: | Data |
| 3,7-dihydroxy-2-(3,4,5-trihydroxyphenyl)chromen-4-one | |
| Chemidplus: | 0000490313 |
References:
| 3,7-dihydroxy-2-(3,4,5-trihydroxyphenyl)chromen-4-one | |
| NIST Chemistry WebBook: | Search Inchi |
| Pubchem (cid): | 5281692 |
| Pubchem (sid): | 134976285 |
Other Information:
| (IUPAC): | Atomic Weights of the Elements 2011 (pdf) |
| Videos: | The Periodic Table of Videos |
| tgsc: | Atomic Weights use for this web site |
| (IUPAC): | Periodic Table of the Elements |
| CHEBI: | View |
| CHEMBL: | View |
| KEGG (GenomeNet): | C10177 |
| HMDB (The Human Metabolome Database): | Search |
| VCF-Online: | VCF Volatile Compounds in Food |
| ChemSpider: | View |
| Wikipedia: | View |
Potential Blenders and core components note
| None Found | ||
Potential Uses:
| None Found |
Occurrence (nature, food, other): note
| found in nature |
Synonyms:
| 4H-1- | benzopyran-4-one, 3,7-dihydroxy-2-(3,4,5-trihydroxyphenyl)- |
| 4H-1- | benzopyran-4-one, 3,7-dihydroxy-2-(3,4,5-trihydroxyphenyl)- (9CI) |
| 5- | deoxymyricetin |
| 3,7- | dihydroxy-2-(3,4,5-trihydroxy-phenyl)-chromen-4-one |
| 3,7- | dihydroxy-2-(3,4,5-trihydroxyphenyl)-4-benzopyrone |
| 3,7- | dihydroxy-2-(3,4,5-trihydroxyphenyl)-4H-1-benzopyran-4-one |
| 3,7- | dihydroxy-2-(3,4,5-trihydroxyphenyl)-4H-chromen-4-one |
| 3,7- | dihydroxy-2-(3,4,5-trihydroxyphenyl)chromen-4-one |
| flavone, 3,3',4',5',7-pentahydroxy- | |
| 5- | hydroxyfisetin |
| norkanugin | |
| 3,3',4',5',7- | pentahydroxyflavone |
