Articles:
angophorol
Notes:
None found
| CAS Number: | 480-43-3 | 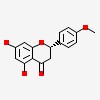 3D/inchi 3D/inchi
|
| ECHA EINECS - REACH Pre-Reg: | 207-551-8 | |
| FDA UNII: | U02X7TF8UA | |
| Nikkaji Web: | J12.391I | |
| MDL: | MFCD00017313 | |
| XlogP3-AA: | 2.70 (est) | |
| Molecular Weight: | 286.28358000 | |
| Formula: | C16 H14 O5 | |
| BioActivity Summary: | listing | |
| NMR Predictor: | Predict (works with chrome or firefox) | |
Category: natural substances and extractives
US / EU / FDA / JECFA / FEMA / FLAVIS / Scholar / Patent Information:
| Google Scholar: | Search |
| Google Books: | Search |
| Google Scholar: with word "volatile" | Search |
| Google Scholar: with word "flavor" | Search |
| Google Scholar: with word "odor" | Search |
| Google Patents: | Search |
| US Patents: | Search |
| EU Patents: | Search |
| Pubchem Patents: | Search |
| PubMed: | Search |
| NCBI: | Search |
Physical Properties:
| Assay: | 95.00 to 100.00 % |
| Food Chemicals Codex Listed: | No |
| Boiling Point: | 539.20 °C. @ 760.00 mm Hg (est) |
| Flash Point: | 402.00 °F. TCC ( 205.60 °C. ) (est) |
| logP (o/w): | 3.840 (est) |
| Soluble in: | |
| water, 109.2 mg/L @ 25 °C (est) | |
Organoleptic Properties:
| Odor and/or flavor descriptions from others (if found). | |
Cosmetic Information:
| None found |
Suppliers:
| Alfa Biotechnology |
| For experimental / research use only. |
| Isosakuranetin 98% |
| BOC Sciences |
| For experimental / research use only. |
| Isosakuranetin 98.5%
Odor: characteristic Use: Isosakuranetin isolated from the fruits of Citrus aurantium L.
antifungal; cytotoxic; |
| Coompo |
| For experimental / research use only. |
| isoSakuranetin from Plants ≥96%
Odor: characteristic Use: Isosakuranetin (ISK) is a plant exudate with known cytotoxic and fungicide properties. When tested on wheat root segments at a concentration of 70µM it inhibited K+ dependent H+ extrusion and net uptake of K+, while leaving the membrane potential (PD) unaltered. Fusicoccin (FC) + ISK treatment resulted in a slight membrane depolarization, while ISK alone did not alter O2 consumption or alternative oxidase activity. ISK did not increase the pyruvate content in incubated root tissues or inhibit Fe2+ uptake. The observed drop in K+ net uptake depended on a decrease in K+ influx into the cell, leading to the suggestion that ISK may act on wheat root segments as an inhibitor of K+ permeation. The lack of proton leakage and membrane disruption by ISK made this compound a weak candidate as a phytoalexin, and suggesting a major role as an allelopathic molecule.
Isosakuranetin produced significant decrease in blood pressure. |
| ExtraSynthese |
| For experimental / research use only. |
| isoSakuranetin (HPLC) ≥99% |
Safety Information:
| Hazards identification | |
| Classification of the substance or mixture | |
| GHS Classification in accordance with 29 CFR 1910 (OSHA HCS) | |
| None found. | |
| GHS Label elements, including precautionary statements | |
| Pictogram | |
| Hazard statement(s) | |
| None found. | |
| Precautionary statement(s) | |
| None found. | |
| Oral/Parenteral Toxicity: | |
| Not determined | |
| Dermal Toxicity: | |
| Not determined | |
| Inhalation Toxicity: | |
| Not determined | |
Safety in Use Information:
| Category: | natural substances and extractives | ||
| Recommendation for isosakuranetin usage levels up to: | |||
| not for fragrance use. | |||
| Recommendation for isosakuranetin flavor usage levels up to: | |||
| not for flavor use. | |||
Safety References:
| EPI System: | View |
| Cancer Citations: | Search |
| Toxicology Citations: | Search |
| EPA ACToR: | Toxicology Data |
| EPA Substance Registry Services (SRS): | Registry |
| Laboratory Chemical Safety Summary : | 160481 |
| National Institute of Allergy and Infectious Diseases: | Data |
| (2S)-5,7-dihydroxy-2-(4-methoxyphenyl)-2,3-dihydrochromen-4-one | |
| Chemidplus: | 0000480433 |
References:
| (2S)-5,7-dihydroxy-2-(4-methoxyphenyl)-2,3-dihydrochromen-4-one | |
| NIST Chemistry WebBook: | Search Inchi |
| Pubchem (cid): | 160481 |
| Pubchem (sid): | 135116440 |
Other Information:
| (IUPAC): | Atomic Weights of the Elements 2011 (pdf) |
| Videos: | The Periodic Table of Videos |
| tgsc: | Atomic Weights use for this web site |
| (IUPAC): | Periodic Table of the Elements |
| CHEBI: | View |
| CHEMBL: | View |
| KEGG (GenomeNet): | C05334 |
| HMDB (The Human Metabolome Database): | Search |
| FooDB: | FDB000610 |
| VCF-Online: | VCF Volatile Compounds in Food |
| ChemSpider: | View |
| Wikipedia: | View |
Potential Blenders and core components note
| None Found | ||
Potential Uses:
| None Found |
Occurrence (nature, food, other): note
| orange fruit Search Trop Picture |
Synonyms:
| angophorol | |
| 4H-1- | benzopyran-4-one, 2,3-dihydro-5,7-dihydroxy-2-(4-methoxyphenyl)-, (2S)- |
| 4H-1- | benzopyran-4-one, 2,3-dihydro-5,7-dihydroxy-2-(4-methoxyphenyl)-, (S)- |
| (S)-2,3- | dihydro-5,7-dihydroxy-2-(4-methoxyphenyl)-4-benzopyrone |
| (S)-2,3- | dihydro-5,7-dihydroxy-2-(4-methoxyphenyl)-4H-1-benzopyran-4-one |
| (2S)-5,7- | dihydroxy-2-(4-methoxyphenyl)-2,3-dihydro-4H-chromen-4-one |
| (2S)-5,7- | dihydroxy-2-(4-methoxyphenyl)-2,3-dihydrochromen-4-one |
| (2S)-5,7- | dihydroxy-2-(4-methoxyphenyl)-3,4-dihydro-2H-1-benzopyran-4-one |
| (2S)-5,7- | dihydroxy-2-(4-methoxyphenyl)chroman-4-one |
| 5,7- | dihydroxy-4'-methoxyflavanone |
| 4'- | methyl naringenin |
| 4'- | methylnaringenin |
| naringenin 4'-methyl ether | |
| iso | sakutanetin |
