Articles:
3-(5,6-dihydro-6,6-dimethyl-2-oxo-2H-pyran-3-yl)-1(3H)-isobenzofuranone
Notes:
None found
| CAS Number: | 1585-68-8 | 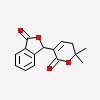 3D/inchi 3D/inchi
|
| Other(deleted CASRN): | 133591-03-4 | |
| FDA UNII: | P61GH0V29Z | |
| Nikkaji Web: | J13.166K | |
| XlogP3-AA: | 2.40 (est) | |
| Molecular Weight: | 258.27338000 | |
| Formula: | C15 H14 O4 | |
| BioActivity Summary: | listing | |
| NMR Predictor: | Predict (works with chrome or firefox) | |
Category: natural substances and extractives
US / EU / FDA / JECFA / FEMA / FLAVIS / Scholar / Patent Information:
| Google Scholar: | Search |
| Google Books: | Search |
| Google Scholar: with word "volatile" | Search |
| Google Scholar: with word "flavor" | Search |
| Google Scholar: with word "odor" | Search |
| Google Patents: | Search |
| US Patents: | Search |
| EU Patents: | Search |
| Pubchem Patents: | Search |
| PubMed: | Search |
| NCBI: | Search |
Physical Properties:
| Assay: | 95.00 to 100.00 % |
| Food Chemicals Codex Listed: | No |
| Boiling Point: | 467.10 °C. @ 760.00 mm Hg (est) |
| Flash Point: | 473.00 °F. TCC ( 245.20 °C. ) (est) |
| logP (o/w): | 2.050 (est) |
| Soluble in: | |
| water, 2022 mg/L @ 25 °C (est) | |
Organoleptic Properties:
| Odor and/or flavor descriptions from others (if found). | |
Cosmetic Information:
| None found |
Suppliers:
| BOC Sciences |
| For experimental / research use only. |
| Catalpalactone
Odor: characteristic Use: Catalpalactone isolated from the wood of Catalpa ovata. It inhibited dopamine biosynthesis by reducing TH and AADC activities and enhanced L-DOPA-induced cytotoxiciy in PC12 cells. |
| Coompo |
| For experimental / research use only. |
| Catalpalactone from Plants ≥96%
Odor: characteristic Use: The effects of catalpalactone on dopamine biosynthesis and L-DOPA-induced cytotoxicity in PC12 cells were investigated. Catalpalactone at 5-30然 decreased intracellular dopamine content with the IC(50) value of 22.1然. Catalpalactone at 5-20然, but not 30然, did not alter cell viability. Catalpalactone at 20然 inhibited tyrosine hydroxylase (TH) and aromatic-l-amino acid decarboxylase (AADC) activities. Catalpalactone also decreased cyclic AMP levels and inhibited TH phosphorylation. In addition, catalpalactone at 20然 reduced the increases in dopamine levels induced by L-DOPA (20-50然). Catalpalactone (5-30然) associated with L-DOPA (50-100然) enhanced L-DOPA-induced cytotoxicity at 48h, which was prevented by N-acetyl-l-cysteine. These results suggest that catalpalactone inhibited dopamine biosynthesis by reducing TH and AADC activities and enhanced L-DOPA-induced cytotoxiciy in PC12 cells. |
Safety Information:
| Hazards identification | |
| Classification of the substance or mixture | |
| GHS Classification in accordance with 29 CFR 1910 (OSHA HCS) | |
| None found. | |
| GHS Label elements, including precautionary statements | |
| Pictogram | |
| Hazard statement(s) | |
| None found. | |
| Precautionary statement(s) | |
| None found. | |
| Oral/Parenteral Toxicity: | |
| Not determined | |
| Dermal Toxicity: | |
| Not determined | |
| Inhalation Toxicity: | |
| Not determined | |
Safety in Use Information:
| Category: | natural substances and extractives | ||
| Recommendation for catalpalactone usage levels up to: | |||
| not for fragrance use. | |||
| Recommendation for catalpalactone flavor usage levels up to: | |||
| not for flavor use. | |||
Safety References:
| EPI System: | View |
| AIDS Citations: | Search |
| Cancer Citations: | Search |
| Toxicology Citations: | Search |
| EPA ACToR: | Toxicology Data |
| EPA Substance Registry Services (SRS): | Registry |
| Laboratory Chemical Safety Summary : | 3014018 |
| National Institute of Allergy and Infectious Diseases: | Data |
| 3-(2,2-dimethyl-6-oxo-3H-pyran-5-yl)-3H-2-benzofuran-1-one | |
| Chemidplus: | 0001585688 |
References:
| 3-(2,2-dimethyl-6-oxo-3H-pyran-5-yl)-3H-2-benzofuran-1-one | |
| NIST Chemistry WebBook: | Search Inchi |
| Pubchem (cid): | 3014018 |
| Pubchem (sid): | 135205113 |
Other Information:
| (IUPAC): | Atomic Weights of the Elements 2011 (pdf) |
| Videos: | The Periodic Table of Videos |
| tgsc: | Atomic Weights use for this web site |
| (IUPAC): | Periodic Table of the Elements |
| CHEBI: | View |
| CHEMBL: | View |
| KEGG (GenomeNet): | C16929 |
| HMDB (The Human Metabolome Database): | Search |
| VCF-Online: | VCF Volatile Compounds in Food |
| ChemSpider: | View |
| Wikipedia: | View |
| EFSA Update of results on the monitoring of furan levels in food: | Read Report |
| EFSA Previous report: Results on the monitoring of furan levels in food: | Read Report |
| EFSA Report of the CONTAM Panel on provisional findings on furan in food: | Read Report |
Potential Blenders and core components note
| None Found | ||
Potential Uses:
| None Found |
Occurrence (nature, food, other): note
| found in nature |
Synonyms:
| 1(3H)-iso | benzofuranone, 3-(5,6-dihydro-6,6-dimethyl-2-oxo-2H-pyran-3-yl)- |
| 3-(5,6- | dihydro-6,6-dimethyl-2-oxo-2H-pyran-3-yl)-1(3H)-isobenzofuranone |
| 3-(6,6- | dimethyl-2-oxo-5,6-dihydro-2H-pyran-3-yl)-2-benzofuran-1(3H)-one |
| 3-(2,2- | dimethyl-6-oxo-3H-pyran-5-yl)-3H-2-benzofuran-1-onee |
