Articles:
15-hydroxylabda-8(17),13-dien-19-oic acid
Notes:
a labdane that induces premature labor.
| CAS Number: | 1909-91-7 | 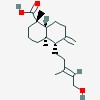 3D/inchi 3D/inchi
|
| Nikkaji Web: | J148.791D | |
| XlogP3-AA: | 4.60 (est) | |
| Molecular Weight: | 320.47264000 | |
| Formula: | C20 H32 O3 | |
| BioActivity Summary: | listing | |
| NMR Predictor: | Predict (works with chrome or firefox) | |
Category: natural substances and extractives
US / EU / FDA / JECFA / FEMA / FLAVIS / Scholar / Patent Information:
| Google Scholar: | Search |
| Google Books: | Search |
| Google Scholar: with word "volatile" | Search |
| Google Scholar: with word "flavor" | Search |
| Google Scholar: with word "odor" | Search |
| Google Patents: | Search |
| US Patents: | Search |
| EU Patents: | Search |
| Pubchem Patents: | Search |
| PubMed: | Search |
| NCBI: | Search |
Physical Properties:
| Assay: | 95.00 to 100.00 % |
| Food Chemicals Codex Listed: | No |
| Boiling Point: | 454.00 to 455.00 °C. @ 760.00 mm Hg (est) |
| Flash Point: | 469.00 °F. TCC ( 242.60 °C. ) (est) |
| logP (o/w): | 4.867 (est) |
| Soluble in: | |
| water, 0.6267 mg/L @ 25 °C (est) | |
Organoleptic Properties:
| Odor and/or flavor descriptions from others (if found). | |
Cosmetic Information:
| None found |
Suppliers:
| BOC Sciences |
| For experimental / research use only. |
| Isocupressic acid 0.965
Odor: characteristic Use: Isocupressic acid isolated from the barks of Araucaria cunninghami. |
| Coompo |
| For experimental / research use only. |
| isoCupressic Acid from Plants ≥96%
Odor: characteristic Use: Isocupressic acid (ICA) induces abortion in pregnant cows when ingested primarily during the last trimester. The objective of this study was to investigate the effects of isocupressic acid on bovine oocyte maturation (in vitro maturation (IVM)-Experiment I) and preimplantation embryo development (in vitro culture (IVC)-Experiment II) using in vitro embryo production techniques and to subsequently evaluate viability and developmental competence of ICA-cultured embryos via embryo transfer to recipient heifers (Experiment III). A complete randomized block experimental design was used. In Experiment I and II, isocupressic acid was added to IVM or IVC media at 0 (TRT1, control), 1.3 (TRT2), and 2.6 microg/ml (TRT3) Results from Experiment I and II indicated that ICA did not inhibit oocyte maturation and did not adversely affect preinpiantation embryo development. Furthermore, results from Experiment II demonstrated that isocupressic acid enhanced bovine preimplantation embryo development in vitro in a dose dependent manner. Subsequently, all but two births were normal as evaluated by post natal veterinary examination. In conclusion, ICA showed no adverse effects on oocyte maturation and preimplantation embryo development in vitro or subsequent viability in vivo using the ICA concentrations and in vitro culture parameters of this study.
Isocupressic acid results in the decrease in progesterone secretion, which affects the angiogenesis of the female reproductive system. The decrease in blood flow and apoptosis of corpus luteum-derived endothelial cells of the uterine tissue results in abortion. However, a more detailed and in-depth study is needed to obtain strong supporting evidence for this hypothesis of Isocupressic acid-induced abortive mechanism in pregnant cows. |
Safety Information:
| Hazards identification | |
| Classification of the substance or mixture | |
| GHS Classification in accordance with 29 CFR 1910 (OSHA HCS) | |
| None found. | |
| GHS Label elements, including precautionary statements | |
| Pictogram | |
| Hazard statement(s) | |
| None found. | |
| Precautionary statement(s) | |
| None found. | |
| Oral/Parenteral Toxicity: | |
| Not determined | |
| Dermal Toxicity: | |
| Not determined | |
| Inhalation Toxicity: | |
| Not determined | |
Safety in Use Information:
| Category: | natural substances and extractives | ||
| Recommendation for isocupressic acid usage levels up to: | |||
| not for fragrance use. | |||
| Recommendation for isocupressic acid flavor usage levels up to: | |||
| not for flavor use. | |||
Safety References:
| EPI System: | View |
| AIDS Citations: | Search |
| Cancer Citations: | Search |
| Toxicology Citations: | Search |
| EPA ACToR: | Toxicology Data |
| EPA Substance Registry Services (SRS): | Registry |
| Laboratory Chemical Safety Summary : | 6438138 |
| National Institute of Allergy and Infectious Diseases: | Data |
| (1S,4aR,5S,8aR)-5-[(E)-5-hydroxy-3-methylpent-3-enyl]-1,4a-dimethyl-6-methylidene-3,4,5,7,8,8a-hexahydro-2H-naphthalene-1-carboxylic acid | |
| Chemidplus: | 0001909917 |
References:
| (1S,4aR,5S,8aR)-5-[(E)-5-hydroxy-3-methylpent-3-enyl]-1,4a-dimethyl-6-methylidene-3,4,5,7,8,8a-hexahydro-2H-naphthalene-1-carboxylic acid | |
| NIST Chemistry WebBook: | Search Inchi |
| Pubchem (cid): | 6438138 |
| Pubchem (sid): | 135074155 |
Other Information:
| (IUPAC): | Atomic Weights of the Elements 2011 (pdf) |
| Videos: | The Periodic Table of Videos |
| tgsc: | Atomic Weights use for this web site |
| (IUPAC): | Periodic Table of the Elements |
| CHEMBL: | View |
| HMDB (The Human Metabolome Database): | Search |
| VCF-Online: | VCF Volatile Compounds in Food |
| ChemSpider: | View |
Potential Blenders and core components note
| None Found | ||
Potential Uses:
| None Found |
Occurrence (nature, food, other): note
| juniper Search Trop Picture | |
| thujopsis dolabrata seed Search Trop Picture |
Synonyms:
| (1S,4aR,5S,8aR)-5-[(3E)-5- | hydroxy-3-methylpent-3-en-1-yl]-1,4a-dimethyl-6-methylidenedecahydronaphthalene-1-carboxylic acid |
| (1S,4aR,5S,8aR)-5-[(E)-5- | hydroxy-3-methylpent-3-enyl]-1,4a-dimethyl-6-methylidene-3,4,5,7,8,8a-hexahydro-2H-naphthalene-1-carboxylic acid |
| 15- | hydroxylabda-8(17),13-dien-19-oic acid |
| 1- | naphthalenecarboxylic acid, decahydro-5-((3E)-5-hydroxy-3-methyl-3-pentenyl)-1,4a-dimethyl-6-methylene-, (1S,4aR,5S,8aR)- |
| 1- | naphthalenecarboxylic acid, decahydro-5-[(3E)-5-hydroxy-3-methyl-3-penten-1-yl]-1,4a-dimethyl-6-methylene-, (1S,4aR,5S,8aR)- |
