Articles:
1-naphthalenecarboxylic acid, 5-[2-(2,5-dihydro-2-oxo-3-furanyl)ethyl]decahydro-1,4a-dimethyl-6-methylene-, methyl ester, (1S,4aR,5S,8aR)-
Notes:
Constit. of the oleorosin of Pinus koraiensis (Korean pine)
| CAS Number: | 31685-80-0 | 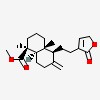 3D/inchi 3D/inchi
|
| Nikkaji Web: | J17.758J | |
| XlogP3-AA: | 4.50 (est) | |
| Molecular Weight: | 346.46690000 | |
| Formula: | C21 H30 O4 | |
| BioActivity Summary: | listing | |
| NMR Predictor: | Predict (works with chrome or firefox) | |
Category: natural substances and extractives
US / EU / FDA / JECFA / FEMA / FLAVIS / Scholar / Patent Information:
| Google Scholar: | Search |
| Google Books: | Search |
| Google Scholar: with word "volatile" | Search |
| Google Scholar: with word "flavor" | Search |
| Google Scholar: with word "odor" | Search |
| Google Patents: | Search |
| US Patents: | Search |
| EU Patents: | Search |
| Pubchem Patents: | Search |
| PubMed: | Search |
| NCBI: | Search |
Physical Properties:
| Assay: | 95.00 to 100.00 % |
| Food Chemicals Codex Listed: | No |
| Boiling Point: | 465.30 °C. @ 760.00 mm Hg (est) |
| Flash Point: | 443.00 °F. TCC ( 228.50 °C. ) (est) |
| logP (o/w): | 4.520 (est) |
| Soluble in: | |
| water, 0.2962 mg/L @ 25 °C (est) | |
Organoleptic Properties:
| Odor and/or flavor descriptions from others (if found). | |
Cosmetic Information:
| None found |
Suppliers:
| BOC Sciences |
| For experimental / research use only. |
| 1-Naphthalenecarboxylicacid,5-[2-(2,5-dihydro-2-oxo-3-furanyl)ethyl]decahydro-1,4a-dimethyl-6-methylene-,methyl ester, (1S,4aR,5S,8aR)- |
| Coompo |
| For experimental / research use only. |
| Pinusolide from Plants ≥96%
Odor: characteristic Use: Pinusolide inhibited 5-lipoxygenase (5-LO)-dependent leukotriene C4 (LTC4) generation in immunoglobulin E (IgE)/Ag-induced bone marrow-derived mast cells (BMMCs) in a concentration-dependent manner. To clarify the action mechanism of pinusolide on the inhibition of LTC4 generation, we examined the effect of pinusolide on phosphorylation of cytosolic phospholipase A2 (cPLA2), as well as translocation phospho-cPLA2 and 5-LO to nucleus. Inhibition of LTC4 generation by pinusolide was accompanied by a decrease in cPLA2 phosphorylation which occurred via a decrease in intracellular Ca2+ influx and blocking the c-Jun N-terminal kinase (JNK) pathways. However, pinusolide had no effect on extracellular signal-regulated kinase (ERK) and p38 mitogen-activated protein (MAP) kinas phosphorylation. Taken together, the present results suggest that potent inhibition of 5-LO dependent LTC4 generation by pinusolide requires both suppression of calcium influx and JNK phosphorylation.
Pinusolide is a potent and specific PAF antagonist in all experimental models as shown in vitro, in vivo, and in animal tests.
Pinusolide and 15-MPA protect neuronal cells from STS-induced apoptosis, probably by preventing the increase in [Ca(2+)]i and cellular oxidation caused by STS, and indicate that they could be used to treat neurodegenerative diseases. |
Safety Information:
| Hazards identification | |
| Classification of the substance or mixture | |
| GHS Classification in accordance with 29 CFR 1910 (OSHA HCS) | |
| None found. | |
| GHS Label elements, including precautionary statements | |
| Pictogram | |
| Hazard statement(s) | |
| None found. | |
| Precautionary statement(s) | |
| None found. | |
| Oral/Parenteral Toxicity: | |
| Not determined | |
| Dermal Toxicity: | |
| Not determined | |
| Inhalation Toxicity: | |
| Not determined | |
Safety in Use Information:
| Category: | natural substances and extractives | ||
| Recommendation for pinusolide usage levels up to: | |||
| not for fragrance use. | |||
| Recommendation for pinusolide flavor usage levels up to: | |||
| not for flavor use. | |||
Safety References:
| EPI System: | View |
| AIDS Citations: | Search |
| Cancer Citations: | Search |
| Toxicology Citations: | Search |
| EPA ACToR: | Toxicology Data |
| EPA Substance Registry Services (SRS): | Registry |
| Laboratory Chemical Safety Summary : | 161721 |
| National Institute of Allergy and Infectious Diseases: | Data |
| methyl (1S,4aR,5S,8aR)-1,4a-dimethyl-6-methylidene-5-[2-(5-oxo-2H-furan-4-yl)ethyl]-3,4,5,7,8,8a-hexahydro-2H-naphthalene-1-carboxylate | |
| Chemidplus: | 0031685800 |
References:
| methyl (1S,4aR,5S,8aR)-1,4a-dimethyl-6-methylidene-5-[2-(5-oxo-2H-furan-4-yl)ethyl]-3,4,5,7,8,8a-hexahydro-2H-naphthalene-1-carboxylate | |
| NIST Chemistry WebBook: | Search Inchi |
| Pubchem (cid): | 161721 |
| Pubchem (sid): | 135118210 |
Other Information:
| (IUPAC): | Atomic Weights of the Elements 2011 (pdf) |
| Videos: | The Periodic Table of Videos |
| tgsc: | Atomic Weights use for this web site |
| (IUPAC): | Periodic Table of the Elements |
| CHEBI: | View |
| CHEMBL: | View |
| HMDB (The Human Metabolome Database): | HMDB35130 |
| FooDB: | FDB013766 |
| VCF-Online: | VCF Volatile Compounds in Food |
| ChemSpider: | View |
| Wikipedia: | View |
| EFSA Update of results on the monitoring of furan levels in food: | Read Report |
| EFSA Previous report: Results on the monitoring of furan levels in food: | Read Report |
| EFSA Report of the CONTAM Panel on provisional findings on furan in food: | Read Report |
Potential Blenders and core components note
| None Found | ||
Potential Uses:
| None Found |
Occurrence (nature, food, other): note
| pinus koraiensis oleorosin Search Trop Picture |
Synonyms:
| (1S-(1alpha,4aalpha,5alpha,8abeta))-5-(2-(2,5- | dihydro-2-oxo-3-furanyl)ethyl) decahydro-1,4a-dimethyl-6-methylene-1-naphthalene carboxylic acid methyl ester |
| methyl (1S,4aR,5S,8aR)-1,4a-dimethyl-6-methylene-5-[2-(2-oxo-2,5-dihydro-3-furanyl)ethyl]decahydro-1-naphthalenecarboxylate | |
| methyl (1S,4aR,5S,8aR)-1,4a-dimethyl-6-methylidene-5-[2-(2-oxo-2,5-dihydrofuran-3-yl)ethyl]decahydronaphthalene-1-carboxylate | |
| methyl (1S,4aR,5S,8aR)-1,4a-dimethyl-6-methylidene-5-[2-(5-oxo-2H-furan-4-yl)ethyl]-3,4,5,7,8,8a-hexahydro-2H-naphthalene-1-carboxylate | |
| 1- | naphthalenecarboxylic acid, 5-(2-(2,5-dihydro-2-oxo-3-furanyl)ethyl)decahydro-1,4a-dimethyl-6-methylene-, methyl ester, (1S-(1a,4aa,5a,8ab))- |
| 1- | naphthalenecarboxylic acid, 5-[2-(2,5-dihydro-2-oxo-3-furanyl)ethyl]decahydro-1,4a-dimethyl-6-methylene-, methyl ester, (1S,4aR,5S,8aR)- |
