Articles:
(+)-3-thujone
Notes:
Thujone is a ketone and a monoterpene that occurs naturally in two diastereomeric forms: (-)-alpha-thujone and (+)-beta-thujone. It has a menthol odor. In addition to (-)-alpha-thujone and (+)-beta-thujone, there are their enantiomeric forms, (+)-alpha-thujone and (-)-beta-thujone. (Wikipedia)
| CAS Number: | 471-15-8 | 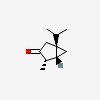 3D/inchi 3D/inchi
|
| FDA UNII: | 8ZI5R3T54Q | |
| Nikkaji Web: | J22.151A | |
| XlogP3-AA: | 2.30 (est) | |
| Molecular Weight: | 152.23672000 | |
| Formula: | C10 H16 O | |
| NMR Predictor: | Predict (works with chrome or firefox) | |
Category: natural substances and extractives
US / EU / FDA / JECFA / FEMA / FLAVIS / Scholar / Patent Information:
| Google Scholar: | Search |
| Google Books: | Search |
| Google Scholar: with word "volatile" | Search |
| Google Scholar: with word "flavor" | Search |
| Google Scholar: with word "odor" | Search |
| Google Patents: | Search |
| US Patents: | Search |
| EU Patents: | Search |
| Pubchem Patents: | Search |
Physical Properties:
| Assay: | 95.00 to 100.00 % |
| Food Chemicals Codex Listed: | No |
| Soluble in: | |
| water, 407.7 mg/L @ 25 °C (est) | |
Organoleptic Properties:
| Odor Description: | herbal warm |
| Odor and/or flavor descriptions from others (if found). | |
Cosmetic Information:
| None found |
Suppliers:
| BOC Sciences |
| For experimental / research use only. |
| THUJONE |
Safety Information:
| Hazards identification | |
| Classification of the substance or mixture | |
| GHS Classification in accordance with 29 CFR 1910 (OSHA HCS) | |
| None found. | |
| GHS Label elements, including precautionary statements | |
| Pictogram | |
| Hazard statement(s) | |
| None found. | |
| Precautionary statement(s) | |
| None found. | |
| Oral/Parenteral Toxicity: | |
| Not determined | |
| Dermal Toxicity: | |
| Not determined | |
| Inhalation Toxicity: | |
| Not determined | |
Safety in Use Information:
| Category: | natural substances and extractives | ||
| IFRA Critical Effect: | Neurotoxicity | ||
| IFRA: | View Standard | ||
| View IFRA Standards Library for complete information. | |||
| Please review Amendment 49 IFRA documentation for complete information. | |||
| IFRA RESTRICTION LIMITS IN THE FINISHED PRODUCT (%): | |||
| Category 1: Products applied to the lips | |||
| 0.11 % | |||
| Category 2: Products applied to the axillae | |||
| 0.21 % | |||
| Category 3: Products applied to the face/body using fingertips | |||
| 0.032 % | |||
| Category 4: Products related to fine fragrance | |||
| 1.40 % | |||
| Category 5: Products applied to the face and body using the hands (palms), primarily leave-on | |||
| Category 5A: Body lotion products applied to the body using the hands (palms), primarily leave-on | |||
| 0.095 % | |||
| Category 5B: Face moisturizer products applied to the face using the hands (palms), primarily leave-on | |||
| 0.032 % | |||
| Category 5C: Hand cream products applied to the hands using the hands (palms), primarily leave-on | |||
| 0.016 % | |||
| Category 5D: Baby Creams, baby Oils and baby talc | |||
| 0.0053 % | |||
| Category 6: Products with oral and lip exposure | |||
| 0.095 % | |||
| Category 7: Products applied to the hair with some hand contact | |||
| Category 7A: Rinse-off products applied to the hair with some hand contact | |||
| 0.24 % | |||
| Category 7B: Leave-on products applied to the hair with some hand contact | |||
| 0.24 % | |||
| Category 8: Products with significant anogenital exposure | |||
| 0.0053 % | |||
| Category 9: Products with body and hand exposure, primarily rinse off | |||
| 0.13 % | |||
| Category 10: Household care products with mostly hand contact | |||
| Category 10A: Household care excluding aerosol products (excluding aerosol/spray products) | |||
| 0.13 % | |||
| Category 10B: Household aerosol/spray products | |||
| 0.22 % | |||
| Category 11: Products with intended skin contact but minimal transfer of fragrance to skin from inert substrate | |||
| Category 11A: Products with intended skin contact but minimal transfer of fragrance to skin from inert substrate without UV exposure | |||
| 0.0053 % | |||
| Category 11B: Products with intended skin contact but minimal transfer of fragrance to skin from inert substrate with potential UV exposure | |||
| 0.0053 % | |||
| Category 12: Products not intended for direct skin contact, minimal or insignificant transfer to skin | |||
| 9.50 % | |||
| Notes: | |||
| IFRA FLAVOR REQUIREMENTS: | |||
Due to the possible ingestion of small amounts of fragrance ingredients from their use in products in Categories 1 and 6, materials must not only comply with IFRA Standards but must also be recognized as safe as a flavoring ingredient as defined by the IOFI Code of Practice (www.iofi.org). For more details see chapter 1 of the Guidance for the use of IFRA Standards. | |||
| Recommendation for (+)-isothujone flavor usage levels up to: | |||
| not for flavor use. | |||
Safety References:
| EPI System: | View |
| EPA ACToR: | Toxicology Data |
| EPA Substance Registry Services (SRS): | Registry |
| Laboratory Chemical Safety Summary : | 91456 |
| National Institute of Allergy and Infectious Diseases: | Data |
| (1S,4S,5R)-4-methyl-1-propan-2-ylbicyclo[3.1.0]hexan-3-one | |
| Chemidplus: | 0000471158 |
References:
| (1S,4S,5R)-4-methyl-1-propan-2-ylbicyclo[3.1.0]hexan-3-one | |
| NIST Chemistry WebBook: | Search Inchi |
| Pubchem (cid): | 91456 |
| Pubchem (cas): | 471-15-8 |
| Pherobase: | View |
Other Information:
| (IUPAC): | Atomic Weights of the Elements 2011 (pdf) |
| Videos: | The Periodic Table of Videos |
| tgsc: | Atomic Weights use for this web site |
| (IUPAC): | Periodic Table of the Elements |
| CHEBI: | View |
| CHEMBL: | View |
| KEGG (GenomeNet): | C20260 |
| HMDB (The Human Metabolome Database): | HMDB36113 |
| FooDB: | FDB014960 |
| VCF-Online: | VCF Volatile Compounds in Food |
| ChemSpider: | View |
Potential Blenders and core components note
| None Found | ||
Potential Uses:
| None Found |
Occurrence (nature, food, other): note
| basil plant Search Trop Picture | |
| ginger rhizome oil Search Trop Picture | |
| hyssop shoot Search Trop Picture | |
| oregano shoot Search Trop Picture | |
| peppermint oil Search Trop Picture | |
| rosemary leaf Search Trop Picture | |
| rosemary shoot Search Trop Picture | |
| sage leaf Search Trop Picture | |
| sage leaf oil Search Trop Picture | |
| sage oil Search Trop Picture | |
| salvia pomifera Search Trop Picture | |
| savory winter savory plant Search Trop Picture | |
| tanacetum argyrophyllum var.argyrophyllum Search Trop Picture |
Synonyms:
| (1S-(1-alpha,4-beta,5-alpha))-4- | methyl-1-(1-methyl ethyl) bicyclo(3.1.0)hexan-3-one |
| (1S,4S,5R)-4- | methyl-1-propan-2-ylbicyclo[3.1.0]hexan-3-one |
| (1S,4S,5R)-(+)-3- | thujanone |
| (+)- | thujone |
| (+)-3- | thujone |
| (+)-beta- | thujone |
| D-iso | thujone |
| dextro- | thujone |
| dextro-iso | thujone |
