Articles:
BCNU
Notes:
Isol. from the common clam Mercenaria mercenaria and from Mercenaria campechiensis
Carmustine is one of the nitrosoureas indicated as palliative therapy as a single agent or in established combination therapy with other approved chemotherapeutic agents in treatment of brain tumors, multiple myeloma, Hodgkin's disease, and non-Hodgkin's lymphomas. Although it is generally agreed that carmustine alkylates DNA and RNA, it is not cross resistant with other alkylators. As with other nitrosoureas, it may also inhibit several key enzymatic processes by carbamoylation of amino acids in proteins.
| CAS Number: | 154-93-8 | 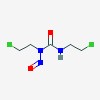 3D/inchi 3D/inchi
|
| Other(deleted CASRN): | 1159711-15-5 | |
| ECHA EINECS - REACH Pre-Reg: | 205-838-2 | |
| FDA UNII: | U68WG3173Y | |
| Nikkaji Web: | J2.968H | |
| Beilstein Number: | 2049744 | |
| MDL: | MFCD00057706 | |
| XlogP3: | 1.50 (est) | |
| Molecular Weight: | 214.05013000 | |
| Formula: | C5 H9 Cl2 N3 O2 | |
| NMR Predictor: | Predict (works with chrome or firefox) | |
Category: natural substances and extractives
US / EU / FDA / JECFA / FEMA / FLAVIS / Scholar / Patent Information:
| Google Scholar: | Search |
| Google Books: | Search |
| Google Scholar: with word "volatile" | Search |
| Google Scholar: with word "flavor" | Search |
| Google Scholar: with word "odor" | Search |
| Google Patents: | Search |
| US Patents: | Search |
| EU Patents: | Search |
| Pubchem Patents: | Search |
| PubMed: | Search |
| NCBI: | Search |
Physical Properties:
| Appearance: | orange-yellow solid (est) |
| Assay: | 95.00 to 100.00 % |
| Food Chemicals Codex Listed: | No |
| Soluble in: | |
| water, 4000 mg/L @ 25 °C (exp) | |
Organoleptic Properties:
| Odor and/or flavor descriptions from others (if found). | |
Cosmetic Information:
| None found |
Suppliers:
| BOC Sciences |
| For experimental / research use only. |
| Carmustine >98%
Odor: characteristic Use: Carmustine is antineoplastic nitrosourea. Carmustine alkylates and cross-links DNA during all phases of the cell cycle, resulting in disruption of DNA function, cell cycle arrest, and apoptosis. This agent also carbamoylates proteins, including DNA |
| Sigma-Aldrich: Sigma |
| For experimental / research use only. |
| Carmustine ≥98% |
Safety Information:
| Preferred SDS: View | |
| Hazards identification | |
| Classification of the substance or mixture | |
| GHS Classification in accordance with 29 CFR 1910 (OSHA HCS) | |
| None found. | |
| GHS Label elements, including precautionary statements | |
| Pictogram | |
| Hazard statement(s) | |
| None found. | |
| Precautionary statement(s) | |
| None found. | |
| Oral/Parenteral Toxicity: | |
|
intravenous-child LDLo 78 mg/kg/52W-I LUNGS, THORAX, OR RESPIRATION: OTHER CHANGES GASTROINTESTINAL: NAUSEA OR VOMITING BLOOD: CHANGES IN BONE MARROW NOT INCLUDED ABOVE Cancer Vol. 42, Pg. 74, 1978. intravenous-dog LDLo 2 mg/kg Cancer Chemotherapy Reports, Part 1. Vol. 57, Pg. 33, 1973. oral-dog LDLo 5 mg/kg Advances in Cancer Research. Vol. 16, Pg. 273, 1972. parenteral-hamster LD10 18 mg/kg Journal of Surgical Oncology. Vol. 15, Pg. 355, 1980. intravenous-human TDLo 6 mg/kg BLOOD: THROMBOCYTOPENIA GASTROINTESTINAL: NAUSEA OR VOMITING BLOOD: LEUKOPENIA Cancer Treatment Reports. Vol. 60, Pg. 709, 1976. intravenous-human TDLo 125 mg/kg BLOOD: LEUKOPENIA BLOOD: THROMBOCYTOPENIA GASTROINTESTINAL: NAUSEA OR VOMITING Advances in Cancer Research. Vol. 16, Pg. 273, 1972. intramuscular-mouse LD50 86300 ug/kg GASTROINTESTINAL: "HYPERMOTILITY, DIARRHEA" BEHAVIORAL: ATAXIA Iyakuhin Kenkyu. Study of Medical Supplies. Vol. 9, Pg. 766, 1978. intraperitoneal-mouse LD50 21260 ug/kg National Cancer Institute Screening Program Data Summary, Developmental Therapeutics Program. Vol. JAN1986 intravenous-mouse LD50 45 mg/kg Proceedings of the Society for Experimental Biology and Medicine. Vol. 118, Pg. 756, 1965. oral-mouse LD50 19 mg/kg GASTROINTESTINAL: "HYPERMOTILITY, DIARRHEA" KIDNEY, URETER, AND BLADDER: URINE VOLUME INCREASED LIVER: "JAUNDICE, OTHER OR UNCLASSIFIED" Toxicology and Applied Pharmacology. Vol. 21, Pg. 405, 1972. unreported-mouse LD50 42 mg/kg Cancer Research. Vol. 46, Pg. 2703, 1986. intramuscular-rat LD50 79600 ug/kg GASTROINTESTINAL: "HYPERMOTILITY, DIARRHEA" BEHAVIORAL: ATAXIA Iyakuhin Kenkyu. Study of Medical Supplies. Vol. 9, Pg. 766, 1978. intraperitoneal-rat LD50 17420 ug/kg National Cancer Institute Screening Program Data Summary, Developmental Therapeutics Program. Vol. JAN1986 intravenous-rat LD50 13800 ug/kg LUNGS, THORAX, OR RESPIRATION: CHRONIC PULMONARY EDEMA GASTROINTESTINAL: ULCERATION OR BLEEDING FROM STOMACH BLOOD: CHANGES IN BONE MARROW NOT INCLUDED ABOVE Oncology. Vol. 37, Pg. 177, 1980. oral-rat LD50 20 mg/kg Journal of Pharmacology and Experimental Therapeutics. Vol. 166, Pg. 104, 1969. | |
| Dermal Toxicity: | |
|
subcutaneous-mouse LD50 24 mg/kg KIDNEY, URETER, AND BLADDER: URINE VOLUME INCREASED GASTROINTESTINAL: "HYPERMOTILITY, DIARRHEA" LIVER: "JAUNDICE, OTHER OR UNCLASSIFIED" Toxicology and Applied Pharmacology. Vol. 21, Pg. 405, 1972. subcutaneous-rat LD50 83200 ug/kg GASTROINTESTINAL: "HYPERMOTILITY, DIARRHEA" BEHAVIORAL: ATAXIA Iyakuhin Kenkyu. Study of Medical Supplies. Vol. 9, Pg. 766, 1978. | |
| Inhalation Toxicity: | |
| Not determined | |
Safety in Use Information:
| Category: | natural substances and extractives | ||
| Recommendation for carmustine usage levels up to: | |||
| not for fragrance use. | |||
| Recommendation for carmustine flavor usage levels up to: | |||
| not for flavor use. | |||
Safety References:
| EPI System: | View |
| ClinicalTrials.gov: | search |
| Daily Med: | search |
| Chemical Carcinogenesis Research Information System: | Search |
| AIDS Citations: | Search |
| Cancer Citations: | Search |
| Toxicology Citations: | Search |
| EPA GENetic TOXicology: | Search |
| EPA ACToR: | Toxicology Data |
| EPA Substance Registry Services (SRS): | Registry |
| Laboratory Chemical Safety Summary : | 2578 |
| National Institute of Allergy and Infectious Diseases: | Data |
| WGK Germany: | 3 |
| 1,3-bis(2-chloroethyl)-1-nitrosourea | |
| Chemidplus: | 0000154938 |
| EPA/NOAA CAMEO: | hazardous materials |
| RTECS: | YS2625000 for cas# 154-93-8 |
References:
| 1,3-bis(2-chloroethyl)-1-nitrosourea | |
| NIST Chemistry WebBook: | Search Inchi |
| Canada Domestic Sub. List: | 154-93-8 |
| Pubchem (cid): | 2578 |
| Pubchem (cas): | 154-93-8 |
Other Information:
| (IUPAC): | Atomic Weights of the Elements 2011 (pdf) |
| Videos: | The Periodic Table of Videos |
| tgsc: | Atomic Weights use for this web site |
| (IUPAC): | Periodic Table of the Elements |
| CHEBI: | View |
| CHEMBL: | View |
| KEGG (GenomeNet): | C06873 |
| HMDB (The Human Metabolome Database): | HMDB14407 |
| FooDB: | FDB012974 |
| VCF-Online: | VCF Volatile Compounds in Food |
| ChemSpider: | View |
| Wikipedia: | View |
Potential Blenders and core components note
| None Found | ||
Potential Uses:
| None Found |
Occurrence (nature, food, other): note
| clam Search PMC Picture |
Synonyms:
| BCNU | |
| carmubris | |
| bis | chloroethyl nitrosourea |
| 1,3-bis(2- | chloroethyl)-1-nitrosourea |
| N,N'-bis(2- | chloroethyl)-N-nitrosourea |
| bis-N,N'-( | chloroethyl)nitrosourea |
