Articles:
Notes:
Adenosine 5'-(Trihydrogen Diphosphate), 3'-(Dihydrogen Phosphate), P'-(3-Hydroxy-4-((3-((2-Mercaptoethyl)Amino)-3-Oxopropyl)Amino)-2,2-Di methyl-4-Oxobutyl) Ester, (R)- Coenzyme A (CoA, CoASH, or HSCoA) is a coenzyme, notable for its role in the synthesis and oxidization of fatty acids, and the oxidation of pyruvate in the citric acid cycle. It is adapted from beta-mercaptoethylamine, panthothenate and adenosine triphosphate. Acetyl-CoA is an important molecule itself. It is the precursor to HMG CoA, which is a vital component in cholesterol and ketone synthesis. Furthermore, it contributes an acetyl group to choline to produce acetylcholine, in a reaction catalysed by choline acetyltransferase. Its main task is conveying the carbon atoms within the acetyl group to the citric acid cycle to be oxidized for energy production. -- Wikipedia [HMDB]
| CAS Number: | 85-61-0 | 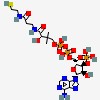 3D/inchi 3D/inchi
|
| Other(deleted CASRN): | 153-46-8 | |
| ECHA EINECS - REACH Pre-Reg: | 201-619-0 | |
| FDA UNII: | SAA04E81UX | |
| Nikkaji Web: | J264.889J | |
| XlogP3: | -5.80 (est) | |
| Molecular Weight: | 767.54040600 | |
| Formula: | C21 H36 N7 O16 P3 S | |
| NMR Predictor: | Predict (works with chrome or firefox) | |
Category: cosmetic ingredient for skin conditioning
US / EU / FDA / JECFA / FEMA / FLAVIS / Scholar / Patent Information:
| Google Scholar: | Search |
| Google Books: | Search |
| Google Scholar: with word "volatile" | Search |
| Google Scholar: with word "flavor" | Search |
| Google Scholar: with word "odor" | Search |
| Google Patents: | Search |
| US Patents: | Search |
| EU Patents: | Search |
| Pubchem Patents: | Search |
| PubMed: | Search |
| NCBI: | Search |
Physical Properties:
| Assay: | 95.00 to 100.00 % |
| Food Chemicals Codex Listed: | No |
| Flash Point: | 32.00 °F. TCC ( 0.00 °C. ) (est) |
| logP (o/w): | -4.358 (est) |
| Soluble in: | |
| water, 2.192e+004 mg/L @ 25 °C (est) | |
Organoleptic Properties:
| Odor and/or flavor descriptions from others (if found). | |
Cosmetic Information:
| CosIng: | cosmetic data |
| Cosmetic Uses: |
skin conditioning |
Suppliers:
| BOC Sciences |
| For experimental / research use only. |
| Coenzyme A Purity >85% |
| George Uhe Company |
| Coenzyme A, Free Acid |
| Glentham Life Sciences |
| Coenzyme A hydrate |
| Santa Cruz Biotechnology |
| For experimental / research use only. |
| Coenzyme A ≥93% |
| Sigma-Aldrich: Sigma |
| For experimental / research use only. |
| Coenzyme A hydrate ≥85% (UV, HPLC) |
Safety Information:
| Preferred SDS: View | |
| Hazards identification | |
| Classification of the substance or mixture | |
| GHS Classification in accordance with 29 CFR 1910 (OSHA HCS) | |
| None found. | |
| GHS Label elements, including precautionary statements | |
| Pictogram | |
| Hazard statement(s) | |
| None found. | |
| Precautionary statement(s) | |
| None found. | |
| Oral/Parenteral Toxicity: | |
| Not determined | |
| Dermal Toxicity: | |
| Not determined | |
| Inhalation Toxicity: | |
| Not determined | |
Safety in Use Information:
| Category: | cosmetic ingredient for skin conditioning | ||
| Recommendation for coenzyme A usage levels up to: | |||
| not for fragrance use. | |||
| Recommendation for coenzyme A flavor usage levels up to: | |||
| not for flavor use. | |||
Safety References:
| EPI System: | View |
| Cancer Citations: | Search |
| Toxicology Citations: | Search |
| EPA Substance Registry Services (TSCA): | 85-61-0 |
| EPA ACToR: | Toxicology Data |
| EPA Substance Registry Services (SRS): | Registry |
| Laboratory Chemical Safety Summary : | 6816 |
| National Institute of Allergy and Infectious Diseases: | Data |
| [[(2R,3S,4R,5R)-5-(6-aminopurin-9-yl)-4-hydroxy-3-phosphonooxyoxolan-2-yl]methoxy-hydroxyphosphoryl][3-hydroxy-2,2-dimethyl-4-oxo-4-[[3-oxo-3-(2-sulfanylethylam | |
| Chemidplus: | 0000085610 |
References:
| [[(2R,3S,4R,5R)-5-(6-aminopurin-9-yl)-4-hydroxy-3-phosphonooxyoxolan-2-yl]methoxy-hydroxyphosphoryl][3-hydroxy-2,2-dimethyl-4-oxo-4-[[3-oxo-3-(2-sulfanylethylam | |
| NIST Chemistry WebBook: | Search Inchi |
| Canada Domestic Sub. List: | 85-61-0 |
| Pubchem (cid): | 6816 |
| Pubchem (sid): | 134972978 |
Other Information:
| (IUPAC): | Atomic Weights of the Elements 2011 (pdf) |
| Videos: | The Periodic Table of Videos |
| tgsc: | Atomic Weights use for this web site |
| (IUPAC): | Periodic Table of the Elements |
| CHEMBL: | View |
| Golm Metabolome Database: | Search |
| Metabolomics Database: | Search |
| UM BBD: | Search |
| KEGG (GenomeNet): | C00010 |
| HMDB (The Human Metabolome Database): | HMDB01423 |
| FooDB: | FDB022614 |
| YMDB (Yeast Metabolome Database): | YMDB00045 |
| ChemSpider: | View |
| Wikipedia: | View |
Potential Blenders and core components note
| None Found | ||
Potential Uses:
| emollients | ||
| solvents |
Occurrence (nature, food, other): note
| not found in nature |
Synonyms:
| CoA-SH | |
| coenzyme A-SH | |
| coenzyme ASH | |
| depot-zeel | |
| propionyl CoA | |
| propionyl coenzyme A | |
| S-propanoate CoA | |
| S-propanoate coenzyme A | |
| S-propionate CoA | |
| S-propionate coenzyme A |

