Articles:
DL-malic acid
Notes:
Mainly used as an acidifier in a wide range of food, such as fruit drinks, jams, sweet and sour sauces, boiled sweets, chewing gum. Occasionally used as a metal ion chelator in wine or in hard water. Flavour enhancer
Malic acid is the active ingredient in many sour or tart foods. Malic acid is found mostly in unripe fruits. Malic acid has two stereoisomeric forms (L- and D-enantiomers), though only the L-isomer exists naturally. (Wikipedia) Acidulant, antioxidant, flavouring agent, flavour enhancer. Not for use in baby foods (GRAS)
| CAS Number: | 6915-15-7 | 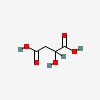 3D/inchi 3D/inchi
|
| Other(deleted CASRN): | 617-48-1 | |
| ECHA EINECS - REACH Pre-Reg: | 230-022-8 | |
| FDA UNII: | 817L1N4CKP | |
| Nikkaji Web: | J3.091K | |
| Beilstein Number: | 1723539 | |
| MDL: | MFCD00064212 | |
| CoE Number: | 17 | |
| XlogP3: | -1.30 (est) | |
| Molecular Weight: | 134.08782000 | |
| Formula: | C4 H6 O5 | |
| BioActivity Summary: | listing | |
| NMR Predictor: | Predict (works with chrome or firefox) | |
Category: flavoring agents and adjuvants, curing and pickling agents, flavor enhancers
US / EU / FDA / JECFA / FEMA / FLAVIS / Scholar / Patent Information:
| Google Scholar: | Search |
| Google Books: | Search |
| Google Scholar: with word "volatile" | Search |
| Google Scholar: with word "flavor" | Search |
| Google Scholar: with word "odor" | Search |
| Perfumer and Flavorist: | Search |
| Google Patents: | Search |
| US Patents: | Search |
| EU Patents: | Search |
| Pubchem Patents: | Search |
| PubMed: | Search |
| NCBI: | Search |
| FDA/DG SANTE Petitions, Reviews, Notices: | |
| 184.1069 | Malic acid (DL-malic acid) View - review |
| EU SANCO | Malic acid View - review |
| JECFA Food Additive: | DL-Malic Acid |
| GSFA Codex: | Malic acid, DL- (296) |
| DG SANTE Food Flavourings: | 08.017 DL-malic acid |
| DG SANTE Food Additives: | DL-malic acid |
| DG SANTE Food Contact Materials: | DL-malic acid |
| FDA Mainterm (SATF): | 617-48-1 ; MALIC ACID |
| FDA Regulation: | |
| FDA PART 184 -- DIRECT FOOD SUBSTANCES AFFIRMED AS GENERALLY RECOGNIZED AS SAFE Subpart B--Listing of Specific Substances Affirmed as GRAS Sec. 184.1069 Malic acid. | |
Physical Properties:
| Appearance: | white crystalline powder (est) |
| Assay: | 99.00 to 100.00 % |
| Food Chemicals Codex Listed: | Yes |
| Melting Point: | 131.00 to 135.00 °C. @ 760.00 mm Hg |
| Boiling Point: | 306.00 to 307.00 °C. @ 760.00 mm Hg |
| Vapor Pressure: | 0.000071 mmHg @ 25.00 °C. (est) |
| Vapor Density: | 4.6 ( Air = 1 ) |
| Flash Point: | 308.00 °F. TCC ( 153.33 °C. ) |
| logP (o/w): | -1.370 (est) |
| Soluble in: | |
| water, 1000000 mg/L @ 20 °C (exp) | |
Organoleptic Properties:
| Odor Type: odorless | |
| Odor Strength: | none |
| Odor Description: at 100.00 %. | odorless |
| Flavor Type: acidic | |
| acidic | |
| Taste Description: | strong acidic |
| Odor and/or flavor descriptions from others (if found). | |
Cosmetic Information:
| None found |
Suppliers:
Safety Information:
| Preferred SDS: View | |
| European information : | |
| Most important hazard(s): | |
| Xn - Harmful. | |
|
R 22 - Harmful if swallowed. R 37/38 - Irritating to respiratory system and skin. R 41 - Risk of serious damage to eyes. S 02 - Keep out of the reach of children. S 20/21 - When using do not eat, drink or smoke. S 22 - Do not breath dust. S 24/25 - Avoid contact with skin and eyes. S 26 - In case of contact with eyes, rinse immediately with plenty of water and seek medical advice. S 36/37/39 - Wear suitable clothing, gloves and eye/face protection. | |
| Hazards identification | |
| Classification of the substance or mixture | |
| GHS Classification in accordance with 29 CFR 1910 (OSHA HCS) | |
| None found. | |
| GHS Label elements, including precautionary statements | |
| Pictogram | |
| Hazard statement(s) | |
| None found. | |
| Precautionary statement(s) | |
| None found. | |
| Oral/Parenteral Toxicity: | |
|
oral-rat LD50 3500 mg/kg (Morgareidge, 1973a) oral-mouse LD50 2660 mg/kg (Morgareidge, 1973b) oral-rabbit LD50 3000 mg/kg (Morgareidge, 1973c) intraperitoneal-mouse LD50 50 mg/kg "Patty's Industrial Hygiene and Toxicology," 3rd rev. ed., Clayton, G.D., and F.E. Clayton, eds., New York, John Wiley & Sons, Inc., 1978-82. Vol. 3 originally pub. in 1979; pub. as 2n rev. ed. in 1985.Vol. 2C, Pg. 4937, 1982. oral-mouse LD50 1600 mg/kg Kodak Company Reports. Vol. #82-0458 intraperitoneal-rat LD50 100 mg/kg "Patty's Industrial Hygiene and Toxicology," 3rd rev. ed., Clayton, G.D., and F.E. Clayton, eds., New York, John Wiley & Sons, Inc., 1978-82. Vol. 3 originally pub. in 1979; pub. as 2n rev. ed. in 1985.Vol. 2C, Pg. 4937, 1982. | |
| Dermal Toxicity: | |
| Not determined | |
| Inhalation Toxicity: | |
| Not determined | |
Safety in Use Information:
| Category: | flavoring agents and adjuvants, curing and pickling agents, flavor enhancers | ||
| Recommendation for malic acid usage levels up to: | |||
| not for fragrance use. | |||
| Maximised Survey-derived Daily Intakes (MSDI-EU): | 13000.00 (μg/capita/day) | ||
Safety References:
| European Food Safety Athority(EFSA): | Flavor usage levels; Subacute, Subchronic, Chronic and Carcinogenicity Studies; Developmental / Reproductive Toxicity Studies; Genotoxicity Studies... |
| European Food Safety Authority (EFSA) reference(s): | |
| Opinion of the Scientific Panel on Food Additives, Flavourings, Processing Aids and Materials in contact with Food (AFC) on a request from the Commission related to - Flavouring Group Evaluation 10: Aliphatic primary and secondary saturated and unsaturated alcohols, aldehydes, acetals, carboxylic acids and esters containing an additional oxygenated functional group and lactones from chemical groups 9, 13 and 30 - (Commission Regulation (EC) No 1565/2000 of 18 July 2000) View page or View pdf | |
| Flavouring Group Evaluation 29 (FGE29)[1] - Substance from the priority list: Vinylbenzene from chemical group 31 - Scientific Opinion of the Panel on Food Additives, Flavourings, Processing Aids and Materials in Contact with Food (AFC) View page or View pdf | |
| 22nd list of substances for food contact materials View page or View pdf | |
| Scientific Report of EFSA on the risk assessment of salts of authorised acids, phenols or alcohols for use in food contact materials [1] View page or View pdf | |
| Scientific Opinion on Flavouring Group Evaluation 92 (FGE.92): Consideration of aliphatic acyclic diols, triols, and related substances evaluated by JECFA (68th meeting) structurally related to aliphatic primary and secondary saturated and unsaturated alcohols, aldehydes, acetals, carboxylic acids and esters containing an additional oxygenated functional group and lactones evaluated by EFSA in FGE.10Rev1 (2009) View page or View pdf | |
| Opinion of the Scientific Panel on food additives, flavourings, processing aids and materials in contact with food (AFC) related to Calcium, Magnesium and Zinc Malate added for nutritional purposes to food supplements as sources for Calcium, Magnesium and Zinc and to Calcium Malate added for nutritional purposes to foods for particular nutritional uses and foods intended for the general population as source for Calcium View page or View pdf | |
| Review of substances/agents that have direct beneficial effect on the environment: mode of action and assessment of efficacy View page or View pdf | |
| Scientific Opinion on Flavouring Group Evaluation 49, Revision 1 (FGE.49Rev1): xanthine alkaloids from the priority list View page or View pdf | |
| EPI System: | View |
| ClinicalTrials.gov: | search |
| Daily Med: | search |
| Chemical Carcinogenesis Research Information System: | Search |
| AIDS Citations: | Search |
| Cancer Citations: | Search |
| Toxicology Citations: | Search |
| EPA Substance Registry Services (TSCA): | 6915-15-7 |
| EPA ACToR: | Toxicology Data |
| EPA Substance Registry Services (SRS): | Registry |
| Laboratory Chemical Safety Summary : | 525 |
| National Institute of Allergy and Infectious Diseases: | Data |
| WGK Germany: | 1 |
| 2-hydroxybutanedioic acid | |
| Chemidplus: | 0006915157 |
| RTECS: | ON7175000 for cas# 6915-15-7 |
References:
| 2-hydroxybutanedioic acid | |
| NIST Chemistry WebBook: | Search Inchi |
| Canada Domestic Sub. List: | 6915-15-7 |
| Pubchem (cid): | 525 |
| Pubchem (sid): | 134987363 |
Other Information:
| (IUPAC): | Atomic Weights of the Elements 2011 (pdf) |
| Videos: | The Periodic Table of Videos |
| tgsc: | Atomic Weights use for this web site |
| (IUPAC): | Periodic Table of the Elements |
| FDA Substances Added to Food (formerly EAFUS): | View |
| FDA Indirect Additives used in Food Contact Substances: | View |
| CHEBI: | View |
| CHEMBL: | View |
| Golm Metabolome Database: | Search |
| Metabolomics Database: | Search |
| KEGG (GenomeNet): | C00711 |
| HMDB (The Human Metabolome Database): | HMDB00744 |
| FooDB: | FDB012047 |
| FDA Listing of Food Additive Status: | View |
| VCF-Online: | VCF Volatile Compounds in Food |
| ChemSpider: | View |
| Wikipedia: | View |
| FAO: | DL-MALIC ACID |
| Formulations/Preparations: •uva ursi, this is volatile oil containing a glucoside, arbutin, tannin, & gallic & malic acids. •grades: technical, active and inactive; fcc. the natural material is levorotatory, but the synthetic material is optically inactive •food grade: powder, fine granular, and granular •granules and powder grades | |
Potential Blenders and core components note
| None Found | ||
Potential Uses:
| None Found |
Occurrence (nature, food, other): note
| allspice fruit Search Trop Picture | |
| apple custard apple plant Search Trop Picture | |
| apricot fruit Search Trop Picture | |
| banana fruit Search Trop Picture | |
| bean fava bean fruit Search Trop Picture | |
| bilberry leaf Search Trop Picture | |
| borage leaf Search Trop Picture | |
| broccoli asparagus broccoli plant Search Trop Picture | |
| brussel sprout leaf Search Trop Picture | |
| buckthorn sea buckthorn fruit Search Trop Picture | |
| buckwheat leaf Search Trop Picture | |
| cabbage leaf Search Trop Picture | |
| carrot root Search Trop Picture | |
| celery root Search Trop Picture | |
| celery shoot Search Trop Picture | |
| chamomile sweet false chamomile plant Search Trop Picture | |
| cherry sour cherry fruit juice Search Trop Picture | |
| chicory plant Search Trop Picture | |
| chive leaf Search Trop Picture | |
| coconut seed Search Trop Picture | |
| corn silk Search Trop Picture | |
| cranberry fruit Search Trop Picture | |
| currant red currant fruit Search Trop Picture | |
| elder black elder fruit Search Trop Picture | |
| fennel seed Search Trop Picture | |
| fig fruit Search Trop Picture | |
| fig fruit juice Search Trop Picture | |
| fig leaf Search Trop Picture | |
| flax seed Search Trop Picture | |
| ginkgo biloba pollen Search Trop Picture | |
| gooseberry fruit Search Trop Picture | |
| grape fruit Search Trop Picture | |
| grape plant Search Trop Picture | |
| kohlrabi stem Search Trop Picture | |
| lettuce leaf Search Trop Picture | |
| lime fruit Search Trop Picture | |
| loquat leaf Search Trop Picture | |
| lupine white lupine seed Search Trop Picture | |
| mandarin fruit Search Trop Picture | |
| mango fruit Search Trop Picture | |
| oat hull husk Search Trop Picture | |
| onion bulb Search Trop Picture | |
| onion leaf Search Trop Picture | |
| orange fruit Search Trop Picture | |
| papaya fruit Search Trop Picture | |
| papaya latex Search Trop Picture | |
| parsley root Search Trop Picture | |
| passion fruit fruit Search Trop Picture | |
| pea seed Search Trop Picture | |
| pear fruit Search Trop Picture | |
| pineapple fruit Search Trop Picture | |
| plum fruit Search Trop Picture | |
| pomegranate fruit Search Trop Picture | |
| pomegranate fruit juice Search Trop Picture | |
| poppy opium poppy latex Search Trop Picture | |
| potato tuber Search Trop Picture | |
| purslane plant Search Trop Picture | |
| quince seed Search Trop Picture | |
| raspberry red raspberry fruit Search Trop Picture | |
| rhubarb plant Search Trop Picture | |
| rose hips fruit Search Trop Picture | |
| roselle flower Search Trop Picture | |
| roselle leaf Search Trop Picture | |
| roselle stem Search Trop Picture | |
| rue leaf Search Trop Picture | |
| sage plant Search Trop Picture | |
| soursop plant Search Trop Picture | |
| soybean seed Search Trop Picture | |
| spinach leaf Search Trop Picture | |
| star fruit fruit Search Trop Picture | |
| sunflower leaf Search Trop Picture | |
| tamarind fruit Search Trop Picture | |
| tamarind leaf Search Trop Picture | |
| tea leaf Search Trop Picture | |
| tomatillo fruit Search Trop Picture | |
| tomato fruit Search Trop Picture | |
| walnut english walnut fruit Search Trop Picture |
Synonyms:
| butanedioic acid, 2-hydroxy- | |
| deoxytetraric acid | |
| (±)-1- | hydroxy-1,2-ethane dicarboxylic acid |
| (±)-1- | hydroxy-1,2-ethanedicarboxylic acid |
| 2- | hydroxy-succinic acid |
| mono | hydroxybernsteinsaeure |
| hydroxybutandisaeure | |
| hydroxybutane dioic acid | |
| 2- | hydroxybutanedioic acid |
| DL- | hydroxybutanedioic acid |
| 2- | hydroxyethane-1,2-dicarboxylic acid |
| hydroxysuccinic acid | |
| (±)- | hydroxysuccinic acid |
| 2- | hydroxysuccinic acid |
| alpha- | hydroxysuccinic acid |
| (±)- | malic acid |
| dextro,laevo- | malic acid |
| DL- | malic acid |
| R,S- | malic acid |
| R,S(±)- | malic acid |
| malic acid DL | |
| malic acid FCC | |
| malic acid FCC fine granular | |
| malic acid FCC powder | |
| malic acid solution | |
| malic acid, (DL) | |
| musashi-no-ringosan | |
| pomalus acid |








