Articles:
2H-1,2,4-benzothiadiazine-7-sulfonamide, 6-chloro-3,4-dihydro-, 1, 1-dioxide
Notes:
a thiazide diuretic often considered the prototypical member of this class. it reduces the reabsorption of electrolytes from the renal tubules. this results in increased excretion of water and electrolytes, including sodium, potassium, chloride, and magnesium. it has been used in the treatment of several disorders including edema, hypertension, diabetes insipidus, and hypoparathyroidism. A thiazide diuretic often considered the prototypical member of this class. It reduces the reabsorption of electrolytes from the renal tubules. This results in increased excretion of water and electrolytes, including sodium, potassium, chloride, and magnesium. It has been used in the treatment of several disorders including edema, hypertension, diabetes insipidus, and hypoparathyroidism. -- Pubchem
Hydrochlorothiazide (Apo-Hydro, Aquazide H, Microzide, Oretic), sometimes abbreviated HCT, HCTZ, or HZT is a popular diuretic drug that acts by inhibiting the kidney's ability to retain water. This reduces the volume of the blood, decreasing peripheral vascular resistance. Chlorothiazide, a carbonic anhydrase inhibitor. --Wikipedia [HMDB]
| CAS Number: | 58-93-5 | 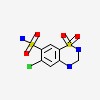 3D/inchi 3D/inchi
|
| Other(deleted CASRN): | 125727-50-6 | |
| ECHA EINECS - REACH Pre-Reg: | 200-403-3 | |
| FDA UNII: | 0J48LPH2TH | |
| Nikkaji Web: | J1.920H | |
| Beilstein Number: | 0625101 | |
| MDL: | MFCD00051765 | |
| XlogP3: | -0.10 (est) | |
| Molecular Weight: | 297.74056000 | |
| Formula: | C7 H8 Cl N3 O4 S2 | |
| BioActivity Summary: | listing | |
| NMR Predictor: | Predict (works with chrome or firefox) | |
Category: pharmaceuticals / chemical synthisis
US / EU / FDA / JECFA / FEMA / FLAVIS / Scholar / Patent Information:
| Google Scholar: | Search |
| Google Books: | Search |
| Google Scholar: with word "volatile" | Search |
| Google Scholar: with word "flavor" | Search |
| Google Scholar: with word "odor" | Search |
| Google Patents: | Search |
| US Patents: | Search |
| EU Patents: | Search |
| Pubchem Patents: | Search |
| PubMed: | Search |
| NCBI: | Search |
Physical Properties:
| Appearance: | white crystalline powder (est) |
| Assay: | 95.00 to 100.00 % |
| Food Chemicals Codex Listed: | No |
| Melting Point: | 274.00 °C. @ 760.00 mm Hg |
| Boiling Point: | 576.96 °C. @ 760.00 mm Hg (est) |
| Flash Point: | 577.00 °F. TCC ( 302.70 °C. ) (est) |
| logP (o/w): | -0.070 |
| Soluble in: | |
| water, 722 mg/L @ 25 °C (exp) | |
| water, 1292 mg/L @ 25 °C (est) | |
Organoleptic Properties:
| Odor and/or flavor descriptions from others (if found). | |
Cosmetic Information:
| None found |
Suppliers:
| BOC Sciences |
| For experimental / research use only. |
| Hydrochlorothiazide >98%
Odor: characteristic Use: Hydrochlorothiazide is a diuretic medication often used to treat high blood pressure and swelling due to fluid build up. |
| Glentham Life Sciences |
| Hydrochlorothiazide |
| Penta International |
| HYDROCHLOROTHIAZIDE USP |
| Sigma-Aldrich |
| For experimental / research use only. |
| Hydrochlorothiazide crystalline |
Safety Information:
| Hazards identification | |
| Classification of the substance or mixture | |
| GHS Classification in accordance with 29 CFR 1910 (OSHA HCS) | |
| None found. | |
| GHS Label elements, including precautionary statements | |
| Pictogram | |
| Hazard statement(s) | |
| None found. | |
| Precautionary statement(s) | |
| None found. | |
| Oral/Parenteral Toxicity: | |
|
intravenous-dog LD50 250 mg/kg "Drug Dosages in Laboratory Animals - A Handbook," Rev. ed., Barnes, C.D., and L.G. Eltherington, Berkeley, Univ. of California Press, 1973Vol. -, Pg. 124, 1973. oral-man TDLo 12857 ug/kg/9D GASTROINTESTINAL: NAUSEA OR VOMITING BEHAVIORAL: CONVULSIONS OR EFFECT ON SEIZURE THRESHOLD American Journal of Medicine. Vol. 70, Pg. 1163, 1981. oral-man TDLo 75 mg/kg/30W-I BEHAVIORAL: CHANGES IN MOTOR ACTIVITY (SPECIFIC ASSAY) BEHAVIORAL: MUSCLE WEAKNESS GASTROINTESTINAL: OTHER CHANGES Rinsho Shinkeigaku. Clinical Neurology. Vol. 17, Pg. 162, 1977. intraperitoneal-mouse LD50 578 mg/kg "Drug Dosages in Laboratory Animals - A Handbook," Rev. ed., Barnes, C.D., and L.G. Eltherington, Berkeley, Univ. of California Press, 1973Vol. -, Pg. 77, 1965. intravenous-mouse LD50 590 mg/kg PERIPHERAL NERVE AND SENSATION: SPASTIC PARALYSIS WITH OR WITHOUT SENSORY CHANGE LUNGS, THORAX, OR RESPIRATION: OTHER CHANGES BEHAVIORAL: CONVULSIONS OR EFFECT ON SEIZURE THRESHOLD Journal of Pharmacology and Experimental Therapeutics. Vol. 134, Pg. 273, 1961. oral-mouse LD50 1175 mg/kg Farmatsevtichnii Zhurnal Vol. (1), Pg. 44, 1983. unreported-mouse LD50 1100 mg/kg Farmatsevtichnii Zhurnal Vol. (5), Pg. 26, 1983. intravenous-rabbit LD50 461 mg/kg "Drug Dosages in Laboratory Animals - A Handbook," Rev. ed., Barnes, C.D., and L.G. Eltherington, Berkeley, Univ. of California Press, 1973Vol. -, Pg. 124, 1973. intraperitoneal-rat LD50 234 mg/kg "Drug Dosages in Laboratory Animals - A Handbook," Rev. ed., Barnes, C.D., and L.G. Eltherington, Berkeley, Univ. of California Press, 1973Vol. -, Pg. 124, 1973. intravenous-rat LD50 990 mg/kg Journal of Pharmacology and Experimental Therapeutics. Vol. 140, Pg. 249, 1963. oral-rat LD50 2750 mg/kg Toxicology and Applied Pharmacology. Vol. 1, Pg. 333, 1959. | |
| Dermal Toxicity: | |
|
subcutaneous-mouse LD50 1470 mg/kg "Drug Dosages in Laboratory Animals - A Handbook," Rev. ed., Barnes, C.D., and L.G. Eltherington, Berkeley, Univ. of California Press, 1973Vol. -, Pg. 124, 1973. subcutaneous-rat LD50 1270 mg/kg "Drug Dosages in Laboratory Animals - A Handbook," Rev. ed., Barnes, C.D., and L.G. Eltherington, Berkeley, Univ. of California Press, 1973Vol. -, Pg. 124, 1973. | |
| Inhalation Toxicity: | |
| Not determined | |
Safety in Use Information:
| Category: | pharmaceuticals / chemical synthisis | ||
| Recommendation for hydrochlorothiazide usage levels up to: | |||
| not for fragrance use. | |||
| Recommendation for hydrochlorothiazide flavor usage levels up to: | |||
| not for flavor use. | |||
Safety References:
| EPI System: | View |
| ClinicalTrials.gov: | search |
| Daily Med: | search |
| Chemical Carcinogenesis Research Information System: | Search |
| AIDS Citations: | Search |
| Cancer Citations: | Search |
| Toxicology Citations: | Search |
| Carcinogenic Potency Database: | Search |
| EPA Substance Registry Services (TSCA): | 58-93-5 |
| EPA ACToR: | Toxicology Data |
| EPA Substance Registry Services (SRS): | Registry |
| Laboratory Chemical Safety Summary : | 3639 |
| National Institute of Allergy and Infectious Diseases: | Data |
| WGK Germany: | 2 |
| 6-chloro-1,1-dioxo-3,4-dihydro-2H-1lamda6,2,4-benzothiadiazine-7-sulfonamide | |
| Chemidplus: | 0000058935 |
| EPA/NOAA CAMEO: | hazardous materials |
| RTECS: | DK9100000 for cas# 58-93-5 |
References:
| 6-chloro-1,1-dioxo-3,4-dihydro-2H-1lamda6,2,4-benzothiadiazine-7-sulfonamide | |
| NIST Chemistry WebBook: | Search Inchi |
| Canada Domestic Sub. List: | 58-93-5 |
| Pubchem (cid): | 3639 |
| Pubchem (sid): | 134972118 |
Other Information:
| (IUPAC): | Atomic Weights of the Elements 2011 (pdf) |
| Videos: | The Periodic Table of Videos |
| tgsc: | Atomic Weights use for this web site |
| (IUPAC): | Periodic Table of the Elements |
| CHEBI: | View |
| CHEMBL: | View |
| KEGG (GenomeNet): | C07041 |
| HMDB (The Human Metabolome Database): | HMDB01928 |
| FooDB: | FDB022745 |
| MedlinePlusSupp: | View |
| ChemSpider: | View |
| Wikipedia: | View |
| Formulations/Preparations: •capsules: 12.5 mg: hydrochlorothiazide capsules, mylan; microzide, watson. oral solution: 50 mg5 ml: hydrochlorothiazide solution, roxane. tablets: 25 mg: aquazide-25, western research; hydrodiuril (scored), merck; oretic, abbott; 50 mg: aquazide-h, western research; ezide, economed; hydrodiuril (scored), merck; 100 mg: hydrochlorothiazide tablets, ivax. •oral tablets, film-coated, 250 mg methylodopa and hydrochlorothiazide 15 mg: aldoril (with propylene glycol), merck; methyldopa and hydrochlorothiazide tablets, endo, major, mylan. 250 mg methyldopa and hydrochlorothiazide 25 mg, aldoril (with propylene glycol), merck; methyldopa and hydrochlorothiazide tablets, endo, major, mylan; 500 mg methyldopa and hydrochlorothiazide 30 mg, aldoril d (with propylene glycol), merck. methyldopa and hydrochlorothiazide •oral capsules, extended-release: 80 mg propranolol hydrochloride and hydrochlorothiazide 50 mg: inderide la, wyeth-ayerst; 120 mg propranolol hyrochloride hydrochlorothiazide 50 mg: inderide la, wyeth-ayerst; 160 mg propranolol hydrochloride and hydrochlorothiazide 50 mg: inderide la, wyeth-ayerst. tablets: 40 mg propranolol hydrochloride and hydrochlorothiazide 25 mg, inderide (scored), wyeth-ayerst; propranolol hydrochloride and hydrochlorothiazide tablets, barr, mylan, purepac, sidmak; 80 mg propranolol hydrochloride and hydrochlorothazide 25 mg: inderide (scored), wyeth-ayerst; propranolol hydrochloride and hydrochlorothiazide tablets, barr, mylan, purepac, sidmak. propranolol hydrochloride and hydrochlorothiazide •oral capsules: 37.5 mg triamterene and hydrochlorothiazide 25 mg: dyazide (with benzyl alcohol and povidone), glaxosmithkline; 50 mg triamterene and hydrochlorothiazide 25 mg: triameterene and hydrochlorothiazide capsules, geneva, ivax, major, martex; oral tablets: 37.5 mg triamterene and hydrochlorothiazide 25 mg: maxzide (scored), bertek; 75 mg triamterene and hydrochlorothiazide 50 mg, maxzide (scored), bertek. triamterene and hydrochlorothiazide (co-triamterzide) •oral capsules: 25 mg with hydralazine hydrochloride 25 mg: hydralazine hydrochloride with hydrochlorothiazide capsules, major, united research; hydra-zide, par; 50 mg with hydralazine hydrochloride 50 mg: hydra-zide, par; 50 mg with hydralazine hydrochloride 100 mg: hydra-zide, par. tablets: 6.25 mg with benazepril 5 mg: lotensin hct (scored), novartis; 12.5 mg with benazepril 10 mg: lotensin hct (scored), novartis; 12.5 mg with benazepril 20 mg: lotensin hct (scored), novartis; 12.5 mg with irbesartan 150 mg, avalide, bristol-myers squibb (also promoted by sanofi-synthelabo); 12.5 mg with irbesartan 300 mg, avalide, briston-meyers squibb (also promoted by sanofi-synthelabo); 12.5 mg with lisinopril 10 mg: prinzide, merck; zestoretic, astrazeneca; 12.5 mg with lisinopril 20 mg: prinzide, merck; zestoretic, astrazeneca; 12.5 mg with valsartan 80 mg: diovan hct, novartis; 12.5 mg with valsartan 160 mg: diovan hct, novartis; 15 mg with hydralazine hydrochloride 25 mg and reserpine 0.1 mg; 25 mg with benazepril 20 mg: lotensin hct (scored), novartis; 25 mg with lisinopril 20 mg: prinzide, merck; zestoretic, astrazeneca. tablets, film-coated: 6.25 mg with bisoprolol fumarate 2.5 mg: ziac, lederle; 6.25 mg with bisoprolol fumarate 5 mg: ziac, lederle; 6.25 mg with bisoprolol fumarate 10 mg: ziac, lederle; 12.5 mg with losartan potassium 50 mg: hyzaar, merck; 12.5 mg with moexipril hydrochloride 7.5 mg: uniretic (scored), schwarz; 12.5 mg with quinapril hydrochloride 10 mg (of quinapril): accuretic (with povidone; scored), parke-davis; 12.5 mg with quinapril hydrochloride 20 mg (of quinapril): accuretic (with povidone; scored), parke-davis; 25 mg with losartan potassium 100 mg: hyzaar, merck; 25 mg with moexipril hydrochloride 15 mg: uniretic (scored), schwarz; 25 mg with quinapril hydrochloride 20 mg (of quinapril): accuretic (with povidone; scored), parke-davis. hydrochlorothiazide combinations •oral tablets: 25 mg captopril and hydrochlorothiazide 15 mg: capozide (scored), bristol-myers squibb; 25 mg captopril and hydrochlorothiazide 25 mg: capozide (scored), bristol-mayers squibb; captopril and hydrochlorothiazide tablets, endo, geneva, ivax, mylan, teva; 50 mg captopril and hydrochlorothiazide 15 mg: capozide (scored), bristol-meyers squibb; captopril and hydrochlorothiazide tablets, endo, geneva, ivax, mylan, teva; 50 mg captopril and hydrochlorothiazide 25 mg: capozide (scored), briston-meyers squibb; captopril and hydrochlorothiazide tablets, endo, geneva, ivax, mylan, teva. captopril and hydrochlorothiazide •oral tablets: 50 mg metoprolol tartrate and hydrochlorothiazide 25 mg: lopressor hct (with povidone; scored), novartis; 100 mg metoprolol tartrate and hydrochlorothiazide 25 mg: lopressor hct (with povidone; scored), novartis; 100 mg metoprolol tartrate and hydrochlorothiazide 50 mg: loperssor hct (with povidone; scored), novartis. metoprolol tartrate and hydrochlorothiazide •tablets, 25, 50, and 100 mg. oral solution, 50 mg5 ml. concentrated oral solution, 100 mgml. •oral tablets: 5 mg of anhydrous amiloride hydrochloride and hydrochlorothiazide 50 mg, moduretic (scored), merck. amiloride hydrochloride and hydrochlorothiazide •oral tablets: 10 mg timolol maleate and hydrochlorothiazide 25 mg: timolide (with povidone; scored), merck. timolol maleate and hydrochlorothiazide •oral tablets: 25 mg spironolacton adn hydrochlorothiazide 25 mg. tablets, film-coated: 25 mg spironolactone and hydrochlorothiazide 25 mg: aldactazide (with povidone), pharmacia; spironolactone and hydrochlorothiazide tablers, major, mutual, mylan, udl, url; 50 mg spironolactone and hydrochlorothiazide 50 mg: aldactazide (with povidone; scored), pharmacia. spironolactone and hydrochlorothiazide •oral tablets: 5 mg enalapril maleate and hydrochlorothiazide 12.5 mg: vaseretic, merck; 10 mg enalapril maleate and hydrochlorothiazide 25 mg: vaseretic, merck. enalapril maleate and hydrochlorothiazide | |
Potential Blenders and core components note
| None Found | ||
Potential Uses:
| None Found |
Occurrence (nature, food, other): note
| not found in nature |
Synonyms:
| accuretic | |
| acuilix | |
| acuretic | |
| aldactazide | |
| aldazida | |
| aldectazide 50/50 | |
| aldoril | |
| apo-hydro | |
| apresazide | |
| apresoline-esidrix | |
| aquarills | |
| aquarius | |
| aquazide H | |
| aquazide-H | |
| avalide | |
| 2H-1,2,4- | benzothiadiazine-7-sulfonamide, 6-chloro-3,4-dihydro-, 1, 1-dioxide |
| bremil | |
| briazide | |
| CAM-AP-ES | |
| caplaril | |
| carozide | |
| catiazida | |
| 6- | chlor-3,4-dihydro-2H-1,2,4-benzothiadiazin-7-sulfonamid-1,1-dioxid |
| chlorizide | |
| 6- | chloro-1,1-dioxo-1,2,3,4-tetrahydro-1lamda*6*-benzo[1,2,4]thiadiazine-7-sulfonic acid amide |
| 6- | chloro-1,1-dioxo-1,2,3,4-tetrahydro-1lamda6-benzo[1,2,4]thiadiazine-7-sulfonic acid amide |
| 6- | chloro-1,1-dioxo-2H,3H,4H-benzo[e]1,2,4-thiadiazine-7-sulfonamide |
| 6- | chloro-1,1-dioxo-3,4-dihydro-2H-1lamda6,2,4-benzothiadiazine-7-sulfonamide |
| 6- | chloro-3,4-dihydro-2H-1,2,4-benzothiadiazine-7-sulfonamide 1,1-dioxide |
| 6- | chloro-3,4-dihydro-2H-benzo[e][1,2,4]thiadiazine-7-sulfonamide 1,1-dioxide |
| 6- | chloro-3,4-dihydro-7-sulfamoyl-2H-1,2, 4-benzothiadiazine 1,1-dioxide |
| 6- | chloro-3,4-dihydro-7-sulfamoyl-2H-1,2,4-benzothiadiazine 1,1-dioxide |
| 6- | chloro-3,4-dihydro-7-sulfamoyl-2H-1,2,4-benzothiadiazine-1,1-dioxide |
| 6- | chloro-7-sulfamoyl-3, 4-dihydro-2H-1,2,4-benzothiadiazine 1,1-dioxide |
| 6- | chloro-7-sulfamoyl-3,4-dihydro-2H-1,2,4-benzothiadiazine 1,1-dioxide |
| 6- | chloro-7-sulfamyl-3,4-dihydro-1,2,4-benzothiadiazine 1,1-dioxide |
| 6- | chloro-7-sulfamyl-3,4-dihydro-1,2,4-benzothiadiazine-1,1-dioxide |
| chlorosulphadil | |
| chlorosulthiadil | |
| chlorsulfonamidodihydrobenzothiadiazine dioxide | |
| chlorzide | |
| chlothia | |
| cidrex | |
| clorana | |
| neo | codema |
| neo- | codema |
| concor plus | |
| condiuren | |
| diaqua | |
| dichlorosal | |
| dichlothiazide | |
| dichlotiazid | |
| dichlotride | |
| diclot ride | |
| diclotride | |
| didral | |
| dihydran | |
| 3,4- | dihydro-6-chloro-7-sulfamoyl-1,2,4-benzothiadiazine-1,1-dioxide |
| 3,4- | dihydro-6-chloro-7-sulfamyl-1,2, 4-benzothiadiazine-1,1-dioxide |
| 3,4- | dihydro-6-chloro-7-sulfamyl-1,2,4-benzothiadiazine-1,1-dioxide |
| dihydrochlorothiazid | |
| 3,4- | dihydrochlorothiazide |
| dihydrochlorurite | |
| dihydroxychlorothiazidum | |
| direma | |
| disalunil | |
| disothiazid | |
| diu-melusin | |
| diurogen | |
| dixidrasi | |
| drenol | |
| dyazide | |
| esidrex | |
| esidrix | |
| esimil | |
| esoidrina | |
| neo | flumen |
| neo- | flumen |
| fluvin | |
| hidril | |
| hidro-niagrin | |
| hidrochlortiazid | |
| hidroronol | |
| hidrosaluretil | |
| hidrotiazida | |
| hyclosid | |
| hydra-zide | |
| hydrap-ES | |
| hydril | |
| hydro par | |
| hydro-aquil | |
| hydro-chlor | |
| hydro-D | |
| hydro-reserp | |
| hydro-ride | |
| hydro-saluric | |
| hydro-T | |
| hydrochloro thiazide | |
| hydrochlorothiazid | |
| hydrochlorthiazide | |
| hydrochlorthiazidum | |
| hydrocot | |
| hydrodiuretic | |
| hydrodiuril | |
| hydrosaluric | |
| hydrothide | |
| hydrozide | |
| hypothaizide | |
| hypothiazid | |
| hytrid | |
| hyzaar | |
| idrotiazide | |
| inderide | |
| indroclor | |
| ivaugan | |
| jen-diril | |
| lopressor HCT | |
| lotensin HCT | |
| manuril | |
| maschitt | |
| maxzide | |
| mazide 25 mg | |
| medozide | |
| megadiuril | |
| microzide | |
| mictrin | |
| mikorten | |
| neo- | minzil |
| modurcen | |
| moduretic | |
| monopril-HCT | |
| natrinax | |
| nefrix | |
| nefrol | |
| newtolide | |
| normozide | |
| novodiurex | |
| oretic | |
| pantemon | |
| panurin | |
| prinzide | |
| quinaretic | |
| raunova plus | |
| ro-hydrazide | |
| saldiuril | |
| sectrazide | |
| selozide | |
| ser-a-gen | |
| ser-ap-es | |
| servithiazid | |
| spironazide | |
| tandiur | |
| thiaretic | |
| thiuretic | |
| thlaretic | |
| timolide | |
| unazid | |
| unipres | |
| uniretic | |
| urodiazin | |
| urozide | |
| vaseretic | |
| vasoretic | |
| vetid rex | |
| vetidrex | |
| viskazide | |
| zestoretic | |
| ziac | |
| zide |


