Articles:
citric acid trisodium salt
Notes:
a key intermediate in metabolism. it is an acid compound found in citrus fruits. the salts of citric acid (citrates) can be used as anticoagulants due to their calcium chelating ability.
| CAS Number: | 68-04-2 | 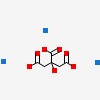 3D/inchi 3D/inchi
|
| ECHA EINECS - REACH Pre-Reg: | 200-675-3 | |
| FDA UNII: | RS7A450LGA | |
| Nikkaji Web: | J1.168A | |
| MDL: | MFCD00012462 | |
| Molecular Weight: | 258.06955784 | |
| Formula: | C6 H5 Na3 O7 | |
| NMR Predictor: | Predict (works with chrome or firefox) | |
Category: preservatives
US / EU / FDA / JECFA / FEMA / FLAVIS / Scholar / Patent Information:
Physical Properties:
| Appearance: | white crystalline powder (est) |
| Assay: | 99.00 to 100.00 % |
| Food Chemicals Codex Listed: | No |
| logP (o/w): | -0.280 (est) |
| Soluble in: | |
| water, 1.2e+005 mg/L @ 25 °C (est) | |
| water, 4.34E+05 mg/L @ C (exp) | |
| Insoluble in: | |
| alcohol | |
Organoleptic Properties:
| Odor Strength: | none |
| Odor Description: at 100.00 %. | odorless |
| Odor and/or flavor descriptions from others (if found). | |
Cosmetic Information:
| CosIng: | cosmetic data |
| Cosmetic Uses: |
buffering agents chelating agents fragrance |
Suppliers:
| ADM |
| Sodium Citrate Anhydrous Powder
Odor: odorless Use: Sodium Citrate Anhydrous is derived from citric acid and is available in either free-flowing colorless granular, fine granular or powder forms. It is odorless and freely soluble in water, but insoluble in alcohol. Storage at room temperature in tightly sealed containers is recommended. Sodium citrate dihydrate is the most broadly used emulsifying salt in sliced processed cheese products. It is commonly used as a buffering agent in combination with citric acid to provide precise pH control required in many food and beverage applications. In meat products it is used as an anticoagulant of fresh livestock blood and as a curing accelerator. The detergent-building and rapid biodegradability characteristics of sodium citrate enable its use as an environmentally acceptable phosphate substitute in a large variety of household cleaning products. |
| ADM |
| Sodium Citrate Anhydrous, USP/FCC |
| Atlantic Chemicals |
| Trisodium Citrate |
| BOC Sciences |
| For experimental / research use only. |
| Sodium citrate,anhydrous 99% |
| Glentham Life Sciences |
| Sodium citrate, anhydrous |
| Graham Chemical |
| Sodium Citrate |
| Jungbunzlauer Suisse |
| Trisodium Citrate Anhydrous
Flavor: characteristic Trisodium citrate is a tribasic salt of citric acid. It is produced by complete neutralisation of citric acid with high purity sodium hydroxide or carbonate and subsequent crystallisation and dehydration.
The common hydrate form, trisodium citrate dihydrate, is widely used in foods, beverages and various technical applications mainly as buffering, sequestering or emulsifying agent, whereas the anhydrous form finds its uses in particular applications such as water sensitive dry blends and instant beverages, surfactants, fragrances as well as in tablets and OTC products.
Jungbunzlauer trisodium citrate anhydrous is manufactured from trisodium citrate dihydrate. Water molecules of the dihydrate crystals are removed by a patented process without destroying the original crystal matrix. The resulting crystals have a porous matrix that can be used as a carrier for inorganic and/or organic substances, especially after being impregnated with liquids. Further, due to the absence of water, the product is not prone to caking. Trisodium citrate anhydrous is also used in applications where excess water is not desired. |
| Lluch Essence |
| SODIUM CITRATE NATURAL |
| M&U International |
| Sodium Citrate, Kosher |
| Penta International |
| SODIUM CITRATE ANHYDROUS GRANULAR USP - FCC |
| Penta International |
| SODIUM CITRATE ANHYDROUS POWDER USP - FCC |
| Penta International |
| TRISODIUM CITRATE |
| Sigma-Aldrich |
| For experimental / research use only. |
| Citric acid trisodium salt ≥98% (GC), anhydrous |
| Tianjin Talent Chemical |
| Trisodium Citrate |
Safety Information:
| Hazards identification | |
| Classification of the substance or mixture | |
| GHS Classification in accordance with 29 CFR 1910 (OSHA HCS) | |
| None found. | |
| GHS Label elements, including precautionary statements | |
| Pictogram | |
| Hazard statement(s) | |
| None found. | |
| Precautionary statement(s) | |
| None found. | |
| Oral/Parenteral Toxicity: | |
|
intraperitoneal-rat LD50 1548 mg/kg BEHAVIORAL: CONVULSIONS OR EFFECT ON SEIZURE THRESHOLD LUNGS, THORAX, OR RESPIRATION: CYANOSIS GASTROINTESTINAL: CHANGES IN STRUCTURE OR FUNCTION OF SALIVARY GLANDS Journal of Pharmacology and Experimental Therapeutics. Vol. 94, Pg. 65, 1948. intravenous-rabbit LD50 449 mg/kg BEHAVIORAL: CONVULSIONS OR EFFECT ON SEIZURE THRESHOLD LUNGS, THORAX, OR RESPIRATION: CYANOSIS GASTROINTESTINAL: CHANGES IN STRUCTURE OR FUNCTION OF SALIVARY GLANDS Journal of Pharmacology and Experimental Therapeutics. Vol. 94, Pg. 65, 1948. intravenous-mouse LD50 170 mg/kg LUNGS, THORAX, OR RESPIRATION: CYANOSIS BEHAVIORAL: CONVULSIONS OR EFFECT ON SEIZURE THRESHOLD GASTROINTESTINAL: CHANGES IN STRUCTURE OR FUNCTION OF SALIVARY GLANDS Journal of Pharmacology and Experimental Therapeutics. Vol. 94, Pg. 65, 1948. intraperitoneal-mouse LD50 1364 mg/kg LUNGS, THORAX, OR RESPIRATION: CYANOSIS BEHAVIORAL: CONVULSIONS OR EFFECT ON SEIZURE THRESHOLD GASTROINTESTINAL: CHANGES IN STRUCTURE OR FUNCTION OF SALIVARY GLANDS Journal of Pharmacology and Experimental Therapeutics. Vol. 94, Pg. 65, 1948. | |
| Dermal Toxicity: | |
| Not determined | |
| Inhalation Toxicity: | |
| Not determined | |
Safety in Use Information:
| Category: | preservatives | ||
| Recommendation for sodium citrate anhydrous usage levels up to: | |||
| not for fragrance use. | |||
| Recommendation for sodium citrate anhydrous flavor usage levels up to: | |||
| not for flavor use. | |||
Safety References:
| EPI System: | View |
| ClinicalTrials.gov: | search |
| NIOSH International Chemical Safety Cards: | search |
| Chemical Carcinogenesis Research Information System: | Search |
| AIDS Citations: | Search |
| Cancer Citations: | Search |
| Toxicology Citations: | Search |
| EPA Substance Registry Services (TSCA): | 68-04-2 |
| EPA ACToR: | Toxicology Data |
| EPA Substance Registry Services (SRS): | Registry |
| Laboratory Chemical Safety Summary : | 6224 |
| National Institute of Allergy and Infectious Diseases: | Data |
| trisodium 2-hydroxypropane-1,2,3-tricarboxylate | |
| Chemidplus: | 0000068042 |
| RTECS: | 68-04-2 |
References:
| trisodium 2-hydroxypropane-1,2,3-tricarboxylate | |
| NIST Chemistry WebBook: | Search Inchi |
| Canada Domestic Sub. List: | 68-04-2 |
| Pubchem (cid): | 6224 |
| Pubchem (sid): | 134971042 |
Other Information:
| (IUPAC): | Atomic Weights of the Elements 2011 (pdf) |
| Videos: | The Periodic Table of Videos |
| tgsc: | Atomic Weights use for this web site |
| (IUPAC): | Periodic Table of the Elements |
| FDA Substances Added to Food (formerly EAFUS): | View |
| CHEBI: | View |
| CHEMBL: | View |
| KEGG (GenomeNet): | D05855 |
| HMDB (The Human Metabolome Database): | Search |
| FDA Listing of Food Additive Status: | View |
| Wikipedia: | View |
| Formulations/Preparations: •sodium citrate is an ingredient in anticoagulant citrate dextrose solution usp; anticoagulant citrate phosphate dextrose solution usp; & sodium citrate & citric acid solution usp. •grades: highest purity, medicinal; pure, commercial, cp; usp; fcc. dihydrate | |
Potential Blenders and core components note
| None Found | ||
Potential Uses:
| buffering agents | ||
| chelating agents |
Occurrence (nature, food, other): note
| not found in nature |
Synonyms:
| citric acid trisodium salt | |
| 2- | hydroxy-1,2,3-propane tricarboxylic acid trisodium salt anhydrous |
| natrocitral | |
| trisodium 2-hydroxy-1,2,3-propane tricarboxylate | |
| trisodium 2-hydroxypropane-1,2,3-tricarboxylate | |
| trisodium citrate |




