Articles:
phosphoric acid sodium salt (1:3)
Notes:
Nutrient supplement, pH control agent, sequestrant, stabiliser, protein modifier, emulsifier in production of processed cheeses, indirect food additive arising from use as a boiler water additive
| CAS Number: | 7601-54-9 | 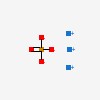 3D/inchi 3D/inchi
|
| Other(deleted CASRN): | 1269628-78-5 | |
| ECHA EINECS - REACH Pre-Reg: | 231-509-8 | |
| FDA UNII: | SX01TZO3QZ | |
| Nikkaji Web: | J3.742G | |
| MDL: | MFCD00003510 | |
| Molecular Weight: | 163.93986984 | |
| Formula: | Na3 O4 P | |
| BioActivity Summary: | listing | |
| NMR Predictor: | Predict (works with chrome or firefox) | |
Category: buffering agents, emulsion stabilizers, sequestrants, acidity regulators
US / EU / FDA / JECFA / FEMA / FLAVIS / Scholar / Patent Information:
Physical Properties:
| Appearance: | white crystalline powder (est) |
| Assay: | 97.00 to 100.00 % |
| Food Chemicals Codex Listed: | No |
| Flash Point: | 32.00 °F. TCC ( 0.00 °C. ) (est) |
| Soluble in: | |
| water, 1e+006 mg/L @ 25 °C (est) | |
| Insoluble in: | |
| alcohol | |
Organoleptic Properties:
| Odor Description: at 100.00 %. | odorless |
| Odor and/or flavor descriptions from others (if found). | |
Cosmetic Information:
| CosIng: | cosmetic data |
| Cosmetic Uses: |
buffering agents chelating agents |
Suppliers:
| American Elements |
| Sodium Phosphate Tribasic Anhydrous 99%
Odor: characteristic Use: Sodium Phosphate Tribasic Anhydrous is generally immediately available in most volumes. High purity, submicron and nanopowder forms may be considered. American Elements produces to many standard grades when applicable, including Mil Spec (military grade); ACS, Reagent and Technical Grade; Food, Agricultural and Pharmaceutical Grade; Optical Grade, USP and EP/BP (European Pharmacopoeia/British Pharmacopoeia) and follows applicable ASTM testing standards. |
| American International Chemical, LLC. |
| TRISODIUM PHOSPHATE ANHYDROUS GRANULAR FCC |
| BOC Sciences |
| For experimental / research use only. |
| Trisodium phosphate tribasic, technical |
| Covalent Chemical |
| Trisodium Phosphate |
| Foodchem International |
| Trisodium Phosphate |
| Glentham Life Sciences |
| Sodium phosphate, anhydrous |
| Graham Chemical |
| Trisodium Phosphate |
| Penta International |
| SODIUM PHOSPHATE TRIBASIC ANHYDROUS NF GRADE |
| Penta International |
| SODIUM PHOSPHATE TRIBASIC ANHYDROUS |
| Sigma-Aldrich: Aldrich |
| For experimental / research use only. |
| Sodium phosphate 96% |
| Silver Fern Chemical |
| Trisodium Phosphate
Odor: characteristic Use: Trisodium Phosphate is commonly used in cleaning agents and detergents, water softeners, textiles, papers, photography, paint removers, food additives, dietary supplements, and as an emulsifier. |
| Tianjin Talent Chemical |
| Trisodium Phosphate |
| Wedor Corporation |
| TRISODIUM PHOSPHATE |
| Zhong Ya Chemical |
| Sodium Phosphate, Tri |
Safety Information:
| Preferred SDS: View | |
| Hazards identification | |
| Classification of the substance or mixture | |
| GHS Classification in accordance with 29 CFR 1910 (OSHA HCS) | |
| None found. | |
| GHS Label elements, including precautionary statements | |
| Pictogram | |
| Hazard statement(s) | |
| None found. | |
| Precautionary statement(s) | |
| None found. | |
| Oral/Parenteral Toxicity: | |
|
intravenous-rabbit LDLo 1580 mg/kg "Abdernalden's Handbuch der Biologischen Arbeitsmethoden." Vol. 4, Pg. 1289, 1935. | |
| Dermal Toxicity: | |
|
skin-rabbit LD > 300 mg/kg Acute Toxicity Data. Journal of the American College of Toxicology, Part B. Vol. 1, Pg. 47, 1990. | |
| Inhalation Toxicity: | |
| Not determined | |
Safety in Use Information:
| Category: | buffering agents, emulsion stabilizers, sequestrants, acidity regulators | ||
| Recommendation for sodium phosphate tribasic usage levels up to: | |||
| not for fragrance use. | |||
| Recommendation for sodium phosphate tribasic flavor usage levels up to: | |||
| not for flavor use. | |||
Safety References:
| European Food Safety Authority (EFSA) reference(s): | |
| Opinion of the Scientific Panel on food additives, flavourings, processing aids and materials in contact with food (AFC) related to Treatment of poultry carcasses with chlorine dioxide, acidified sodium chlorite, trisodium phosphate and peroxyacids View page or View pdf | |
| Assessment of one published review on health risks associated with phosphate additives in food View page or View pdf | |
| Re-evaluation of phosphoric acid–phosphates – di-, tri- and polyphosphates (E 338–341, E 343, E 450–452) as food additives and the safety of proposed extension of use View page or View pdf | |
| EPI System: | View |
| NIOSH International Chemical Safety Cards: | search |
| Chemical Carcinogenesis Research Information System: | Search |
| AIDS Citations: | Search |
| Cancer Citations: | Search |
| Toxicology Citations: | Search |
| EPA Substance Registry Services (TSCA): | 7601-54-9 |
| EPA ACToR: | Toxicology Data |
| EPA Substance Registry Services (SRS): | Registry |
| Laboratory Chemical Safety Summary : | 24243 |
| National Institute of Allergy and Infectious Diseases: | Data |
| WGK Germany: | 1 |
| trisodium phosphate | |
| Chemidplus: | 0007601549 |
| EPA/NOAA CAMEO: | hazardous materials |
| RTECS: | 7601-54-9 |
References:
| trisodium phosphate | |
| NIST Chemistry WebBook: | Search Inchi |
| Canada Domestic Sub. List: | 7601-54-9 |
| Pubchem (cid): | 24243 |
| Pubchem (sid): | 134987258 |
Other Information:
| (IUPAC): | Atomic Weights of the Elements 2011 (pdf) |
| Videos: | The Periodic Table of Videos |
| tgsc: | Atomic Weights use for this web site |
| (IUPAC): | Periodic Table of the Elements |
| FDA Substances Added to Food (formerly EAFUS): | View |
| FDA Indirect Additives used in Food Contact Substances: | View |
| CHEBI: | View |
| CHEMBL: | View |
| KEGG (GenomeNet): | D09000 |
| HMDB (The Human Metabolome Database): | Search |
| FooDB: | FDB013349 |
| Export Tariff Code: | 2835.23.0000 |
| FDA Listing of Food Additive Status: | View |
| ChemSpider: | View |
| Wikipedia: | View |
| Formulations/Preparations: •trisodium phosphate, 1-4% in crest fluoride gel and crest fluoride toothpaste. •anhydrous, technical grades: anhydrous and crystalline, granular and powder food grades. •grade: commercial, high purity, cp chemically pure - a grade designation signifying a minimum of impurities, but not 100% purity, fcc (anhydrous), anhydrous salt | |
Potential Blenders and core components note
| None Found | ||
Potential Uses:
| buffering agents | ||
| chelating agents |
Occurrence (nature, food, other): note
| not found in nature |
Synonyms:
| phosphoric acid sodium salt (1:3) | |
| phosphoric acid trisodium salt | |
| sodium phosphate tribasic | |
| sodium phosphate, tribasic | |
| sodium tertiary phosphate | |
| trisodium orthophosphate | |
| trisodium phosphate | |
| TSP |



