Articles:
[5R-(5a,5ab,8aa,9a)]-5,8,8a,9-tetrahydro-9-hydroxy-5-(3,4,5-trimethoxyphenyl)furo[3',4':6,7]naphtho[2,3-d]-1,3-dioxol-6(5aH)-one
Notes:
a lignan (lignans) found in podophyllin resin from the roots of podophyllum plants. it is a potent spindle poison, toxic if taken internally, and has been used as a cathartic. it is very irritating to skin and mucous membranes, has keratolytic actions, has been used to treat warts and keratoses, and may have antineoplastic properties, as do some of its congeners and derivatives.
| CAS Number: | 518-28-5 | 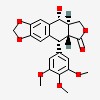 3D/inchi 3D/inchi
|
| Other(deleted CASRN): | 11016-28-7 | |
| ECHA EINECS - REACH Pre-Reg: | 208-250-4 | |
| FDA UNII: | L36H50F353 | |
| Nikkaji Web: | J6.582J | |
| Beilstein Number: | 0099163 | |
| MDL: | MFCD00075290 | |
| XlogP3: | 2.00 (est) | |
| Molecular Weight: | 414.41094000 | |
| Formula: | C22 H22 O8 | |
| BioActivity Summary: | listing | |
| NMR Predictor: | Predict (works with chrome or firefox) | |
Category: natural substances and extractives
US / EU / FDA / JECFA / FEMA / FLAVIS / Scholar / Patent Information:
| Google Scholar: | Search |
| Google Books: | Search |
| Google Scholar: with word "volatile" | Search |
| Google Scholar: with word "flavor" | Search |
| Google Scholar: with word "odor" | Search |
| Google Patents: | Search |
| US Patents: | Search |
| EU Patents: | Search |
| Pubchem Patents: | Search |
| PubMed: | Search |
| NCBI: | Search |
Physical Properties:
| Appearance: | white crystalline powder (est) |
| Assay: | 95.00 to 100.00 % |
| Food Chemicals Codex Listed: | No |
| Melting Point: | 183.00 °C. @ 760.00 mm Hg |
| Boiling Point: | 597.00 to 598.00 °C. @ 760.00 mm Hg (est) |
| Flash Point: | 410.00 °F. TCC ( 210.20 °C. ) (est) |
| logP (o/w): | 2.010 |
| Soluble in: | |
| water, 154.9 mg/L @ 25 °C (est) | |
Organoleptic Properties:
| Odor and/or flavor descriptions from others (if found). | |
Cosmetic Information:
| None found |
Suppliers:
| Alfa Biotechnology |
| For experimental / research use only. |
| Podophyllotoxin 98% |
| AuNutra® Industries |
| Podophyllatoxin P.E. |
| BOC Sciences |
| For experimental / research use only. |
| Podophyllotoxin > 95%
Odor: characteristic Use: Podophyllotoxin, a kind of non-alkaloid toxin lignan extracted from the roots and rhizomes of Podophyllum plant, has been shown to inhibit the growth of various carcinoma cells. It is a potent inhibitor of microtubule assembly and DNA topoisomerase II |
| Coompo |
| For experimental / research use only. |
| Podophyllotoxin from Plants ≥98%
Odor: characteristic Use: Among the plethora of physiological activities and potential medicinal and agricultural applications, the antineoplastic and antiviral properties of podophyllotoxin congeners and their derivatives are arguably the most eminent from a pharmacological perspective.
Podophyllotoxin is an antimitotic. It acts by preventing viral wart cells from dividing and multiplying. Eventually all the wart cells die and new healthy cells grow in their place. In the 1930s, doctors in New Orleans started using podophyllotoxin for the treatment of genital warts. By 1942 the first description of this practice was published in medical literature. |
| ExtraSynthese |
| For experimental / research use only. |
| (-)-Podophyllotoxin (HPLC) ≥99% |
| Glentham Life Sciences |
| Podophyllotoxin |
| Shaanxi Y-Herb Biotechnology |
| Pure Podophyllotoxin Monomer Powder Podophyllum Peltatum Podophyllum Hexandrum Extract |
| Sigma-Aldrich: Sigma |
| For experimental / research use only. |
| Podophyllotoxin |
Safety Information:
| Preferred SDS: View | |
| Hazards identification | |
| Classification of the substance or mixture | |
| GHS Classification in accordance with 29 CFR 1910 (OSHA HCS) | |
| None found. | |
| GHS Label elements, including precautionary statements | |
| Pictogram | |
| Hazard statement(s) | |
| None found. | |
| Precautionary statement(s) | |
| None found. | |
| Oral/Parenteral Toxicity: | |
|
intravenous-rat LD50 8.70 mg/kg LUNGS, THORAX, OR RESPIRATION: DYSPNEA GASTROINTESTINAL: NAUSEA OR VOMITING BEHAVIORAL: SOMNOLENCE (GENERAL DEPRESSED ACTIVITY) Federation Proceedings, Federation of American Societies for Experimental Biology. Vol. 7, Pg. 249, 1948. intraperitoneal-rat LD50 15 mg/kg LUNGS, THORAX, OR RESPIRATION: DYSPNEA BEHAVIORAL: SOMNOLENCE (GENERAL DEPRESSED ACTIVITY) GASTROINTESTINAL: NAUSEA OR VOMITING Federation Proceedings, Federation of American Societies for Experimental Biology. Vol. 7, Pg. 249, 1948. intravenous-rabbit LD50 5 mg/kg LUNGS, THORAX, OR RESPIRATION: DYSPNEA SKIN AND APPENDAGES (SKIN): CUTANEOUS SENSITIZATION (EXPERIMENTAL): AFTER TOPICAL EXPOSURE BEHAVIORAL: SOMNOLENCE (GENERAL DEPRESSED ACTIVITY) Federation Proceedings, Federation of American Societies for Experimental Biology. Vol. 7, Pg. 249, 1948. oral-mouse LD50 100 mg/kg PCT Vol. #86-04062 intravenous-mouse LD50 56 mg/kg U.S. Army Armament Research & Development Command, Chemical Systems Laboratory, NIOSH Exchange Chemicals. Vol. NX#01679 intraperitoneal-mouse LD50 30 mg/kg Arzneimittel-Forschung. Drug Research. Vol. 11, Pg. 327, 1961. intramuscular-cat LD50 4 mg/kg SKIN AND APPENDAGES (SKIN): CUTANEOUS SENSITIZATION (EXPERIMENTAL): AFTER TOPICAL EXPOSURE LUNGS, THORAX, OR RESPIRATION: DYSPNEA BEHAVIORAL: SOMNOLENCE (GENERAL DEPRESSED ACTIVITY) Federation Proceedings, Federation of American Societies for Experimental Biology. Vol. 7, Pg. 249, 1948. intravenous-cat LD50 1700 ug/kg GASTROINTESTINAL: NAUSEA OR VOMITING LUNGS, THORAX, OR RESPIRATION: DYSPNEA BEHAVIORAL: SOMNOLENCE (GENERAL DEPRESSED ACTIVITY) Federation Proceedings, Federation of American Societies for Experimental Biology. Vol. 7, Pg. 249, 1948. parenteral-frog LDLo 152 mg/kg BEHAVIORAL: STIFFNESS Archiv fuer Experimentelle Pathologie und Pharmakologie. Vol. 28, Pg. 32, 1891. intramuscular-rat LD50 3 mg/kg BEHAVIORAL: SOMNOLENCE (GENERAL DEPRESSED ACTIVITY) GASTROINTESTINAL: NAUSEA OR VOMITING LUNGS, THORAX, OR RESPIRATION: DYSPNEA Federation Proceedings, Federation of American Societies for Experimental Biology. Vol. 7, Pg. 249, 1948. intravenous-rat LD50 8700 ug/kg LUNGS, THORAX, OR RESPIRATION: DYSPNEA GASTROINTESTINAL: NAUSEA OR VOMITING BEHAVIORAL: SOMNOLENCE (GENERAL DEPRESSED ACTIVITY) Federation Proceedings, Federation of American Societies for Experimental Biology. Vol. 7, Pg. 249, 1948. | |
| Dermal Toxicity: | |
|
subcutaneous-rat LD50 8 mg/kg BEHAVIORAL: SOMNOLENCE (GENERAL DEPRESSED ACTIVITY) LUNGS, THORAX, OR RESPIRATION: DYSPNEA LUNGS, THORAX, OR RESPIRATION: RESPIRATORY DEPRESSION Proceedings of the Society for Experimental Biology and Medicine. Vol. 77, Pg. 269, 1951. skin-rat LD50 500 mg/kg PCT Vol. #86-04062 skin-rabbit LD50 200 mg/kg PCT Vol. #86-04062 subcutaneous-cat LDLo 300 ug/kg GASTROINTESTINAL: NAUSEA OR VOMITING BEHAVIORAL: CONVULSIONS OR EFFECT ON SEIZURE THRESHOLD Archiv fuer Experimentelle Pathologie und Pharmakologie. Vol. 28, Pg. 32, 1891. subcutaneous-chicken LDLo 6250 ug/kg GASTROINTESTINAL: "HYPERMOTILITY, DIARRHEA" BEHAVIORAL: MUSCLE CONTRACTION OR SPASTICITY) Archiv fuer Experimentelle Pathologie und Pharmakologie. Vol. 28, Pg. 32, 1891. subcutaneous-dog LDLo 1 mg/kg GASTROINTESTINAL: NAUSEA OR VOMITING BEHAVIORAL: CONVULSIONS OR EFFECT ON SEIZURE THRESHOLD Archiv fuer Experimentelle Pathologie und Pharmakologie. Vol. 28, Pg. 32, 1891. subcutaneous-mouse LDLo 24600 ug/kg Toxicon. Vol. 8, Pg. 85, 1970. | |
| Inhalation Toxicity: | |
| Not determined | |
Safety in Use Information:
| Category: | natural substances and extractives | ||
| Recommendation for podophyllotoxin usage levels up to: | |||
| not for fragrance use. | |||
| Recommendation for podophyllotoxin flavor usage levels up to: | |||
| not for flavor use. | |||
Safety References:
| EPI System: | View |
| ClinicalTrials.gov: | search |
| Daily Med: | search |
| Chemical Carcinogenesis Research Information System: | Search |
| AIDS Citations: | Search |
| Cancer Citations: | Search |
| Toxicology Citations: | Search |
| EPA GENetic TOXicology: | Search |
| EPA ACToR: | Toxicology Data |
| EPA Substance Registry Services (SRS): | Registry |
| Laboratory Chemical Safety Summary : | 10607 |
| National Institute of Allergy and Infectious Diseases: | Data |
| WGK Germany: | 3 |
| (5R,5aR,8aR,9R)-5-hydroxy-9-(3,4,5-trimethoxyphenyl)-5a,6,8a,9-tetrahydro-5H-[2]benzofuro[5,6-f][1,3]benzodioxol-8-one | |
| Chemidplus: | 0000518285 |
| RTECS: | LV2500000 for cas# 518-28-5 |
References:
| (5R,5aR,8aR,9R)-5-hydroxy-9-(3,4,5-trimethoxyphenyl)-5a,6,8a,9-tetrahydro-5H-[2]benzofuro[5,6-f][1,3]benzodioxol-8-one | |
| NIST Chemistry WebBook: | Search Inchi |
| Pubchem (cid): | 10607 |
| Pubchem (sid): | 134976487 |
Other Information:
| (IUPAC): | Atomic Weights of the Elements 2011 (pdf) |
| Videos: | The Periodic Table of Videos |
| tgsc: | Atomic Weights use for this web site |
| (IUPAC): | Periodic Table of the Elements |
| CHEBI: | View |
| CHEMBL: | View |
| KEGG (GenomeNet): | C10874 |
| HMDB (The Human Metabolome Database): | Search |
| Export Tariff Code: | 2932.90.4100 |
| MedlinePlusSupp: | View |
| VCF-Online: | VCF Volatile Compounds in Food |
| ChemSpider: | View |
| Wikipedia: | View |
| EFSA Update of results on the monitoring of furan levels in food: | Read Report |
| EFSA Previous report: Results on the monitoring of furan levels in food: | Read Report |
| EFSA Report of the CONTAM Panel on provisional findings on furan in food: | Read Report |
| Formulations/Preparations: topical: gel: 0.5%, condylox, (watson); solution: 0.5%, condylox (with alcohol 95% lactic acid and sodium lactate), (watson). | |
Potential Blenders and core components note
| None Found | ||
Potential Uses:
| None Found |
Occurrence (nature, food, other): note
| found in nature |
Synonyms:
| condyline | |
| condylox | |
| (10R,11R,15R,16R)-16- | hydroxy-10-(3,4,5-trimethoxyphenyl)-4,6,13-trioxatetracyclo[7.7.0.03,7.011,15]hexadeca-1(9),2,7-trien-12-one |
| (5R,5aR,8aR,9R)-9- | hydroxy-5-(3,4,5-trimethoxy-phenyl)-5,8,8a,9-tetrahydro-5aH-furo[3',4':6,7]naphtho[2,3-d][1,3]dioxol-6-one |
| (5R,5aR,8aR,9R)-9- | hydroxy-5-(3,4,5-trimethoxyphenyl)-5,5a,8a,9-tetrahydrofuro[3',4':6,7]naphtho[2,3-d][1,3]dioxol-6(8H)-one |
| (5R,5aR,8aR,9R)-9- | hydroxy-5-(3,4,5-trimethoxyphenyl)-5,8,8a,9-tetrahydrofuro[3',4':6,7]naphtho[2,3-d][1,3]dioxol-6(5aH)-one |
| (5R,9R,5aR,8aR)-9- | hydroxy-5-(3,4,5-trimethoxyphenyl)-5,8,9,5a,8a-pentahydro-2H-isobenzofurano[5',6'-2,1]benzo[4,5-d]1,3-dioxolan-6-one |
| (5R,5aR,8aR,9R)-5- | hydroxy-9-(3,4,5-trimethoxyphenyl)-5a,6,8a,9-tetrahydro-5H-[2]benzofuro[5,6-f][1,3]benzodioxol-8-one |
| podofilox | |
| podophilox | |
| podophyllinic acid lactone | |
| podophyllotoxin (8CI) | |
| podophyllotoxin 7 | |
| (5R,5aR,8aR,9R)-5,8,8a,9- | tetrahydro-9-hydroxy-5-(3,4,5-trimethoxyphenyl)-furo(3',4':6,7)naphtho[2,3-d]-1,3-dioxol-6(5aH)-one |
| (5R,5aR,8aR,9R)-5,8,8a,9- | tetrahydro-9-hydroxy-5-(3,4,5-trimethoxyphenyl)furo(3',4':6,7)naphtho(2,3-d)-1,3-dioxol-6(5aH)-one |
| (5R,5aR,8aR,9R)-5,8,8a,9- | tetrahydro-9-hydroxy-5-(3,4,5-trimethoxyphenyl)furo[3',4':6,7]naphtho[2,3-d]-1,3-dioxol-6(5aH)-one |
| [5R-(5a,5ab,8aa,9a)]-5,8,8a,9- | tetrahydro-9-hydroxy-5-(3,4,5-trimethoxyphenyl)furo[3',4':6,7]naphtho[2,3-d]-1,3-dioxol-6(5aH)-one |
| warticon |

