Articles:
(13-alpha,14-alpha)-14,19-dihydro-12,13-dihydroxy-20-norcrotalanan-11,15-dione
Notes:
Hepatotoxin. Causative agent of much seneciosis, e.g. accidental poisoning by S. by weed residues in bread, and characterised by venoocculosive disease
| CAS Number: | 315-22-0 | 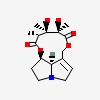 3D/inchi 3D/inchi
|
| FDA UNII: | 73077K8HYV | |
| Nikkaji Web: | J107.104A | |
| Beilstein Number: | 0048732 | |
| MDL: | MFCD00084656 | |
| XlogP3-AA: | -0.70 (est) | |
| Molecular Weight: | 325.36151000 | |
| Formula: | C16 H23 N O6 | |
| BioActivity Summary: | listing | |
| NMR Predictor: | Predict (works with chrome or firefox) | |
Category: natural substances and extractives
US / EU / FDA / JECFA / FEMA / FLAVIS / Scholar / Patent Information:
| Google Scholar: | Search |
| Google Books: | Search |
| Google Scholar: with word "volatile" | Search |
| Google Scholar: with word "flavor" | Search |
| Google Scholar: with word "odor" | Search |
| Google Patents: | Search |
| US Patents: | Search |
| EU Patents: | Search |
| Pubchem Patents: | Search |
| PubMed: | Search |
| NCBI: | Search |
Physical Properties:
| Appearance: | colorless prisms (est) |
| Assay: | 95.00 to 100.00 % |
| Food Chemicals Codex Listed: | No |
| Boiling Point: | 537.30 °C. @ 760.00 mm Hg (est) |
| Flash Point: | 534.00 °F. TCC ( 278.70 °C. ) (est) |
| logP (o/w): | -0.370 (est) |
| Soluble in: | |
| water, 9.019e+005 mg/L @ 25 °C (est) | |
Organoleptic Properties:
| Odor and/or flavor descriptions from others (if found). | |
Cosmetic Information:
| None found |
Suppliers:
| Alfa Biotechnology |
| For experimental / research use only. |
| Monocrotaline; Crotaline 98% |
| BOC Sciences |
| For experimental / research use only. |
| monoCROTALINE >98%
Odor: characteristic Use: Crotaline a natural ligand exhibits dose-dependent cytotoxicity with potent antineoplastic activity.Crotaline produced a positive result in the liver at a non-hepatotoxic lower dose level
anti-cancer |
| ExtraSynthese |
| For experimental / research use only. |
| monoCrotaline TLC ≥90% |
| Glentham Life Sciences |
| Crotaline |
| Santa Cruz Biotechnology |
| For experimental / research use only. |
| monoCrotaline ≥98% |
| Sigma-Aldrich |
| For experimental / research use only. |
| monoCrotaline analytical standard |
| Sigma-Aldrich: Sigma |
| For experimental / research use only. |
| Crotaline |
Safety Information:
| Preferred SDS: View | |
| Hazards identification | |
| Classification of the substance or mixture | |
| GHS Classification in accordance with 29 CFR 1910 (OSHA HCS) | |
| None found. | |
| GHS Label elements, including precautionary statements | |
| Pictogram | |
| Hazard statement(s) | |
| None found. | |
| Precautionary statement(s) | |
| None found. | |
| Oral/Parenteral Toxicity: | |
|
unreported-mammal (species unspecified) LDLo 60 mg/kg Pharmacological Reviews. Vol. 22, Pg. 429, 1970. intraperitoneal-mouse LD50 259 mg/kg Toxicology and Applied Pharmacology. Vol. 59, Pg. 424, 1981. intravenous-mouse LD50 261 mg/kg Journal of Pharmacology and Experimental Therapeutics. Vol. 75, Pg. 78, 1942. intravenous-rat LD50 92 mg/kg Journal of Pharmacology and Experimental Therapeutics. Vol. 83, Pg. 265, 1945. oral-rat LD50 66 mg/kg LIVER: "HEPATITIS (HEPATOCELLULAR NECROSIS), DIFFUSE" Toxicology and Applied Pharmacology. Vol. 18, Pg. 387, 1971. intraperitoneal-rat LDLo 130 mg/kg LIVER: OTHER CHANGES LUNGS, THORAX, OR RESPIRATION: OTHER CHANGES Chemico-Biological Interactions. Vol. 12, Pg. 299, 1976. | |
| Dermal Toxicity: | |
|
subcutaneous-rat LD50 60 mg/kg Toxicology and Applied Pharmacology. Vol. 23, Pg. 470, 1972. | |
| Inhalation Toxicity: | |
| Not determined | |
Safety in Use Information:
| Category: | natural substances and extractives | ||
| Recommendation for monocrotaline usage levels up to: | |||
| not for fragrance use. | |||
| Recommendation for monocrotaline flavor usage levels up to: | |||
| not for flavor use. | |||
Safety References:
| European Food Safety Authority (EFSA) reference(s): | |
| Dietary exposure assessment to pyrrolizidine alkaloids in the European population View page or View pdf | |
| EPI System: | View |
| Chemical Carcinogenesis Research Information System: | Search |
| AIDS Citations: | Search |
| Cancer Citations: | Search |
| Toxicology Citations: | Search |
| Carcinogenic Potency Database: | Search |
| EPA GENetic TOXicology: | Search |
| EPA ACToR: | Toxicology Data |
| EPA Substance Registry Services (SRS): | Registry |
| Laboratory Chemical Safety Summary : | 9415 |
| National Institute of Allergy and Infectious Diseases: | Data |
| WGK Germany: | 3 |
| Chemidplus: | 0000315220 |
| RTECS: | QB3140000 for cas# 315-22-0 |
References:
| NIST Chemistry WebBook: | Search Inchi |
| Pubchem (cid): | 9415 |
| Pubchem (sid): | 134974959 |
Other Information:
| (IUPAC): | Atomic Weights of the Elements 2011 (pdf) |
| Videos: | The Periodic Table of Videos |
| tgsc: | Atomic Weights use for this web site |
| (IUPAC): | Periodic Table of the Elements |
| CHEBI: | View |
| CHEMBL: | View |
| KEGG (GenomeNet): | C10350 |
| HMDB (The Human Metabolome Database): | HMDB34363 |
| FooDB: | FDB012736 |
| Export Tariff Code: | 2939.90.1000 |
| VCF-Online: | VCF Volatile Compounds in Food |
| ChemSpider: | View |
| Wikipedia: | View |
Potential Blenders and core components note
| None Found | ||
Potential Uses:
| None Found |
Occurrence (nature, food, other): note
| crotalaria spectabilis Search Trop Picture |
Synonyms:
| mono | crotalin |
| (13-alpha,14-alpha)-14,19- | dihydro-12,13-dihydroxy-20-norcrotalanan-11,15-dione |
| 12-beta,13-beta- | dihydroxy-12-alpha,13-alpha,14-alpha-trimethylcrotal-1-enine |
| 20- | norcrotalanan-11,15-dione, 14,19-dihydro-12,13-dihydroxy-, (13alpha,14alpha)- |
| (2,3,4-gh) | pyrrolizine-2,6(3H)-dione, (4,5,8,10,12,13,13a,13b)-octahydro-4,5-dihydroxy-3,4,5-trimethyl-2H-(1,6)dioxacycloundecino- |

