Articles:
1-methyl-7-methoxy-3,4-dihydro-b-carboline
Notes:
a beta-carboline alkaloid isolated from seeds of peganum. Alkaloid from Passiflora incarnata (maypops)
| CAS Number: | 304-21-2 | 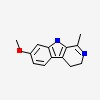 3D/inchi 3D/inchi
|
| ECHA EINECS - REACH Pre-Reg: | 206-152-6 | |
| FDA UNII: | CN58I4TOET | |
| Nikkaji Web: | J11.607F | |
| Beilstein Number: | 0207310 | |
| MDL: | MFCD00004955 | |
| XlogP3-AA: | 1.20 (est) | |
| Molecular Weight: | 214.26778000 | |
| Formula: | C13 H14 N2 O | |
| BioActivity Summary: | listing | |
| NMR Predictor: | Predict (works with chrome or firefox) | |
Category: natural substances and extractives
US / EU / FDA / JECFA / FEMA / FLAVIS / Scholar / Patent Information:
| Google Scholar: | Search |
| Google Books: | Search |
| Google Scholar: with word "volatile" | Search |
| Google Scholar: with word "flavor" | Search |
| Google Scholar: with word "odor" | Search |
| Google Patents: | Search |
| US Patents: | Search |
| EU Patents: | Search |
| Pubchem Patents: | Search |
| PubMed: | Search |
| NCBI: | Search |
Physical Properties:
| Assay: | 95.00 to 100.00 % |
| Food Chemicals Codex Listed: | No |
| Melting Point: | 239.00 to 241.00 °C. @ 760.00 mm Hg |
| Boiling Point: | 426.42 °C. @ 760.00 mm Hg (est) |
| Flash Point: | 413.00 °F. TCC ( 211.70 °C. ) (est) |
| logP (o/w): | 2.251 (est) |
| Soluble in: | |
| water, 4.051 mg/L @ 25 °C (est) | |
Organoleptic Properties:
| Odor and/or flavor descriptions from others (if found). | |
Cosmetic Information:
| None found |
Suppliers:
| Alfa Biotechnology |
| For experimental / research use only. |
| Vasicin 98% |
| BOC Sciences |
| For experimental / research use only. |
| Harmaline 0.985
Odor: characteristic Use: Harmaline comes from the herbs of Peganum harmala L., which is a psychoactive indole found naturally in certain plants. Its stimulating activities are achieved, in part, through inhibition of monoamine oxidases.
Central nervous system stimulants; antifungal; antibacterial |
| Coompo |
| For experimental / research use only. |
| Harmaline from Plants ≥96% |
| ExtraSynthese |
| For experimental / research use only. |
| Harmaline (HPLC) ≥90% |
| Sigma-Aldrich: Aldrich |
| For experimental / research use only. |
| Harmaline |
| TCI AMERICA |
| For experimental / research use only. |
| Harmaline >98.0%(HPLC)(T) |
Safety Information:
| Preferred SDS: View | |
| Hazards identification | |
| Classification of the substance or mixture | |
| GHS Classification in accordance with 29 CFR 1910 (OSHA HCS) | |
| None found. | |
| GHS Label elements, including precautionary statements | |
| Pictogram | |
| Hazard statement(s) | |
| None found. | |
| Precautionary statement(s) | |
| None found. | |
| Oral/Parenteral Toxicity: | |
|
intravenous-rabbit LDLo 20 mg/kg SENSE ORGANS AND SPECIAL SENSES: MYDRIASIS (PUPILLARY DILATION): EYE BEHAVIORAL: ATAXIA BEHAVIORAL: EXCITEMENT Neuropharmacology. Vol. 10, Pg. 15, 1971. | |
| Dermal Toxicity: | |
|
subcutaneous-mouse LD50 120 mg/kg Psychopharmacology Service Center, Bulletin. Vol. 2, Pg. 17, 1963. subcutaneous-rat LD50 120 mg/kg Veterinary and Human Toxicology. Vol. 33, Pg. 276, 1991. | |
| Inhalation Toxicity: | |
| Not determined | |
Safety in Use Information:
| Category: | natural substances and extractives | ||
| Recommendation for harmaline usage levels up to: | |||
| not for fragrance use. | |||
| Recommendation for harmaline flavor usage levels up to: | |||
| not for flavor use. | |||
Safety References:
| EPI System: | View |
| Cancer Citations: | Search |
| Toxicology Citations: | Search |
| EPA ACToR: | Toxicology Data |
| EPA Substance Registry Services (SRS): | Registry |
| Laboratory Chemical Safety Summary : | 5280951 |
| National Institute of Allergy and Infectious Diseases: | Data |
| WGK Germany: | 3 |
| 7-methoxy-1-methyl-3,4-dihydro-2H-pyrido[3,4-b]indole | |
| Chemidplus: | 0000304212 |
| RTECS: | UU9800000 for cas# 304-21-2 |
References:
| 7-methoxy-1-methyl-3,4-dihydro-2H-pyrido[3,4-b]indole | |
| NIST Chemistry WebBook: | Search Inchi |
| Pubchem (cid): | 5280951 |
| Pubchem (sid): | 134973776 |
Other Information:
| (IUPAC): | Atomic Weights of the Elements 2011 (pdf) |
| Videos: | The Periodic Table of Videos |
| tgsc: | Atomic Weights use for this web site |
| (IUPAC): | Periodic Table of the Elements |
| CHEBI: | View |
| CHEMBL: | View |
| KEGG (GenomeNet): | C06536 |
| HMDB (The Human Metabolome Database): | HMDB30310 |
| FooDB: | FDB002148 |
| Export Tariff Code: | 2939.80.0000 |
| VCF-Online: | VCF Volatile Compounds in Food |
| ChemSpider: | View |
| Wikipedia: | View |
Potential Blenders and core components note
| None Found | ||
Potential Uses:
| None Found |
Occurrence (nature, food, other): note
| apricot wild apricot Search Trop Picture | |
| rue wild rue seeds Search Trop Picture |
Synonyms:
| 4,9- | dihydro-7-methoxy-1-methyl-3H-pyrido(3,4-b)indole |
| 3,4- | dihydro-7-methoxy-1-methyl-9-pyrid(3,4-b)indole |
| 3,4- | dihydro-7-methoxy-1-methyl-9-pyrid[3,4-b]indole |
| 3,4- | dihydro-7-methoxy-1-methyl-9-pyrido(3,4-b)indole |
| dihydroharmine | |
| 3,4- | dihydroharmine |
| harmidine | |
| harmine, dihydro- | |
| 7- | methoxy-1-methyl-3,4-dihydro-2H-pyrido[3,4-b]indole |
| 7- | methoxy-1-methyl-4,9-dihydro-3H-b-carboline |
| 1- | methyl-7-methoxy-3,4-dihydro-b-carboline |
| O- | methylharmalol |
| 3H- | pyrido(3,4-b)indole, 4,9-dihydro-7-methoxy-1-methyl- |
| 2H- | pyrido[3,4-b]indole, 3,4-dihydro-7-methoxy-1-methyl- (9CI) |
| 3H- | pyrido[3,4-b]indole, 4, 9-dihydro-7-methoxy-1-methyl- |
| 3H- | pyrido[3,4-b]indole, 4,9-dihydro-7-methoxy-1-methyl- |

