|
Category: flavoring agents, food additives
US / EU / FDA / JECFA / FEMA / FLAVIS / Scholar / Patent Information:
| Google Scholar: | Search |
| Google Books: | Search |
| Google Scholar: with word "volatile" | Search |
| Google Scholar: with word "flavor" | Search |
| Google Scholar: with word "odor" | Search |
| Google Patents: | Search |
| US Patents: | Search |
| EU Patents: | Search |
| Pubchem Patents: | Search |
| PubMed: | Search |
| NCBI: | Search |
| JECFA Food Additive: | Citric Acid |
| GSFA Codex: | Citric acid (330) |
| FDA Mainterm (SATF): | 77-92-9 ; CITRIC ACID |
| FDA Regulation: | FDA PART 131 -- MILK AND CREAM
Subpart B--Requirements for Specific Standardized Milk and Cream
Sec. 131.111 Acidified milk.
FDA PART 131 -- MILK AND CREAM
Subpart B--Requirements for Specific Standardized Milk and Cream
Sec. 131.112 Cultured milk.
FDA PART 133 -- CHEESES AND RELATED CHEESE PRODUCTS
Subpart B--Requirements for Specific Standardized Cheese and Related Products
Sec. 133.123 Cold-pack and club cheese.
FDA PART 133 -- CHEESES AND RELATED CHEESE PRODUCTS
Subpart B--Requirements for Specific Standardized Cheese and Related Products
Sec. 133.124 Cold-pack cheese food.
FDA PART 133 -- CHEESES AND RELATED CHEESE PRODUCTS
Subpart B--Requirements for Specific Standardized Cheese and Related Products
Sec. 133.129 Dry curd cottage cheese.
FDA PART 133 -- CHEESES AND RELATED CHEESE PRODUCTS
Subpart B--Requirements for Specific Standardized Cheese and Related Products
Sec. 133.169 Pasteurized process cheese.
FDA PART 133 -- CHEESES AND RELATED CHEESE PRODUCTS
Subpart B--Requirements for Specific Standardized Cheese and Related Products
Sec. 133.173 Pasteurized process cheese food.
FDA PART 133 -- CHEESES AND RELATED CHEESE PRODUCTS
Subpart B--Requirements for Specific Standardized Cheese and Related Products
Sec. 133.178 Pasteurized neufchatel cheese spread with other foods.
FDA PART 133 -- CHEESES AND RELATED CHEESE PRODUCTS
Subpart B--Requirements for Specific Standardized Cheese and Related Products
Sec. 133.179 Pasteurized process cheese spread.
FDA PART 145 -- CANNED FRUITS
Subpart B--Requirements for Specific Standardized Canned Fruits
Sec. 145.134 Canned preserved figs.
FDA PART 145 -- CANNED FRUITS
Subpart B--Requirements for Specific Standardized Canned Fruits
Sec. 145.145 Canned grapefruit.
FDA PART 146 -- CANNED FRUIT JUICES
Subpart B--Requirements for Specific Standardized Canned Fruit Juices and Beverages
Sec. 146.187 Canned prune juice.
FDA PART 150 -- FRUIT BUTTERS, JELLIES, PRESERVES, AND RELATED PRODUCTS
Subpart B--Requirements for Specific Standardized Fruit Butters, Jellies, Preserves, and Related Products
Sec. 150.141 Artificially sweetened fruit jelly.
FDA PART 150 -- FRUIT BUTTERS, JELLIES, PRESERVES, AND RELATED PRODUCTS
Subpart B--Requirements for Specific Standardized Fruit Butters, Jellies, Preserves, and Related Products
Sec. 150.161 Artificially sweetened fruit preserves and jams.
FDA PART 155 -- CANNED VEGETABLES
Subpart B--Requirements for Specific Standardized Canned Vegetables
Sec. 155.130 Canned corn.
FDA PART 161 -- FISH AND SHELLFISH
Subpart B--Requirements for Specific Standardized Fish and Shellfish
Sec. 161.190 Canned tuna.
FDA PART 163 -- CACAO PRODUCTS
Subpart B--Requirements for Specific Standardized Cacao Products
Sec. 163.110 Cacao nibs.
FDA PART 163 -- CACAO PRODUCTS
Subpart B--Requirements for Specific Standardized Cacao Products
Sec. 163.110 Cacao nibs.
FDA PART 163 -- CACAO PRODUCTS
Subpart B--Requirements for Specific Standardized Cacao Products
Sec. 163.112 Breakfast cocoa.
FDA PART 169 -- FOOD DRESSINGS AND FLAVORINGS
Subpart B--Requirements for Specific Standardized Food Dressings and Flavorings
Sec. 169.115 French dressing.
FDA PART 169 -- FOOD DRESSINGS AND FLAVORINGS
Subpart B--Requirements for Specific Standardized Food Dressings and Flavorings
Sec. 169.140 Mayonnaise.
FDA PART 169 -- FOOD DRESSINGS AND FLAVORINGS
Subpart B--Requirements for Specific Standardized Food Dressings and Flavorings
Sec. 169.150 Salad dressing.
FDA PART 172 -- FOOD ADDITIVES PERMITTED FOR DIRECT ADDITION TO FOOD FOR HUMAN CONSUMPTION
Subpart H--Other Specific Usage Additives
Sec. 172.755 Stearyl monoglyceridyl citrate.
FDA PART 172 -- FOOD ADDITIVES PERMITTED FOR DIRECT ADDITION TO FOOD FOR HUMAN CONSUMPTION
Subpart I--Multipurpose Additives
Sec. 172.861 Cocoa butter substitute from coconut oil, palm kernel oil, or both oils.
FDA PART 173 -- SECONDARY DIRECT FOOD ADDITIVES PERMITTED IN FOOD FOR HUMAN CONSUMPTION
Subpart B--Enzyme Preparations and Microorganisms
Sec. 173.160 Candida guilliermondii.
FDA PART 173 -- SECONDARY DIRECT FOOD ADDITIVES PERMITTED IN FOOD FOR HUMAN CONSUMPTION
Subpart B--Enzyme Preparations and Microorganisms
Sec. 173.165 Candida lipolytica.
FDA PART 173 -- SECONDARY DIRECT FOOD ADDITIVES PERMITTED IN FOOD FOR HUMAN CONSUMPTION
Subpart C--Solvents, Lubricants, Release Agents and Related Substances
Sec. 173.280 Solvent extraction process for citric acid.
FDA PART 178 -- INDIRECT FOOD ADDITIVES: ADJUVANTS, PRODUCTION AIDS, AND SANITIZERS
Subpart B--Substances Utilized To Control the Growth of Microorganisms
Sec. 178.1010 Sanitizing solutions.
FDA PART 184 -- DIRECT FOOD SUBSTANCES AFFIRMED AS GENERALLY RECOGNIZED AS SAFE
Subpart B--Listing of Specific Substances Affirmed as GRAS
Sec. 184.1033 Citric acid.
FDA PART 73 -- LISTING OF COLOR ADDITIVES EXEMPT FROM CERTIFICATION
Subpart A--Foods
Sec. 73.85 Caramel.
|
Physical Properties:
| Appearance: | white powder (est) |
| Assay: | 95.00 to 100.00 %
|
| Food Chemicals Codex Listed: | No |
| Boiling Point: | 558.50 °C. @ 760.00 mm Hg (est)
|
| Flash Point: | 557.00 °F. TCC ( 291.60 °C. ) (est)
|
| Soluble in: |
| | water, 1e+006 mg/L @ 25 °C (est) |
Organoleptic Properties:
| |
| Odor and/or flavor descriptions from others (if found). |
| |
| |
Cosmetic Information:
Suppliers:
Safety Information:
| Preferred SDS: View |
| |
| Hazards identification |
| |
| Classification of the substance or mixture |
| GHS Classification in accordance with 29 CFR 1910 (OSHA HCS) |
| None found. |
| GHS Label elements, including precautionary statements |
| |
| Pictogram | |
| |
| Hazard statement(s) |
| None found. |
| Precautionary statement(s) |
| None found. |
| Oral/Parenteral Toxicity: |
intraperitoneal-rat LD50 375 mg/kg
National Technical Information Service. Vol. AD-A121-876
|
| Dermal Toxicity: |
|
Not determined
|
| Inhalation Toxicity: |
|
Not determined
|
Safety in Use Information:
| Category: | flavoring agents, food additives |
| Recommendation for citric acid monohydrate usage levels up to: | | | not for fragrance use.
|
| |
Safety References:
References:
| | 2-hydroxypropane-1,2,3-tricarboxylic acid;hydrate |
| NIST Chemistry WebBook: | Search Inchi |
| Pubchem (cid): | 22230 |
| Pubchem (sid): | 134987918 |
Other Information:
Potential Blenders and core components notePotential Uses:
Occurrence (nature, food, other): noteSynonyms:
| | citric acid hydrate | | | citric acid, monohydrate | | 2- | hydroxypropane-1,2,3-tricarboxylic acid hydrate | | 2- | hydroxypropane-1,2,3-tricarboxylic acid, hydrate | | 2- | hydroxypropane-1,2,3-tricarboxylic acid;hydrate | | 1,2,3- | propane tricarboxylic acid | | 1,2,3- | propanetricarboxylic acid, 2-hydroxy-, monohydrate |
Articles:
|
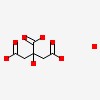 3D/inchi
3D/inchi






